Determination of Trace Nickel in Red Mud by Fluorescence Quenching of CdTe Quantum Dots
-
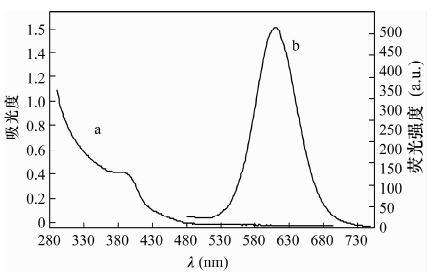
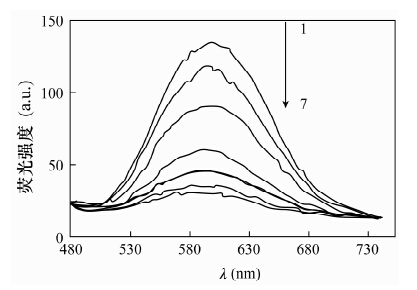
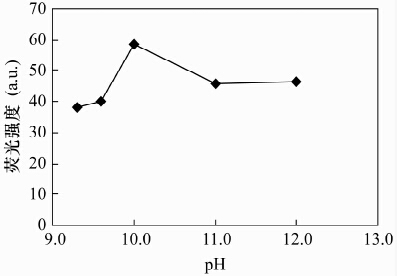
摘要: CdTe量子点具有荧光强度高和稳定性好的优点, 应用CdTe量子点荧光猝灭法分析基体复杂的样品, 需要有效分离对测定干扰的金属元素和高效富集待测元素。本文以巯基乙酸(TGA)作稳定剂, 采用水相合成法制备了巯基乙酸修饰的CdTe量子点, 基于镍离子在pH=10.0硼砂缓冲溶液中对CdTe量子点的荧光具有较强猝灭作用, 建立了一种测定赤泥中痕量镍的荧光光度方法。荧光猝灭反应的最佳实验条件为:CdTe量子点的浓度3.0×10-4 mol/L, 反应温度为室温, 反应时间10 min, 在此条件下镍离子浓度在2.0×10-7~7.8×10-5 mol/L范围内与CdTe量子点的相对荧光强度呈良好的线性关系; 方法检出限为1.5×10-7 mol/L。本方法针对Fe3+、Co2+、Cu2+等主要基体金属元素允许量低的问题, 采用25%的2-羟基-4-仲辛氧基二苯甲酮肟(N530) 磺化煤油萃取回收滤液中的Ni2+, 镍回收率达到99%以上; 赤泥中痕量镍的测定结果与催化光度法相符, 加标回收率为98.3%-104.2%。Abstract: CdTe Quantum Dots (QDs) have the advantages of high fluorescence intensity and good stability. Using CdTe QDs as fluorescence probes to analyze samples containing complex substrates, the interference of metal elements must be effectively separated, and elements whose contents are lower than detection limits must be efficiently enriched. Thioglycolic acid (TGA) modified CdTe QDs of high fluorescence were prepared in aqueous solution using thioglycolic acid as a stabilizer. A new fluorophotometric method for the determination of trace nickel in red mud was developed based on the strong quenching effect that CdTe QDs fluorescence had been quenched by nickel ion in borax buffer solution (pH was 10.0). The suitable experimental conditions were as follows: CdTe quantum dot concentration was 3.0×10-4 mol/L, reaction temperature was room temperature, and reaction time was 10 min. The relative fluorescence intensity of CdTe QDs was proportional to the concentration of nickel ions that ranges from 2.0×10-7 to 7.8×10-5mol/L, and the detection limit was 1.5×10-7 mol/L. In order to substrate the main lower metal elements such as Fe3+, Co2+, and Cu2+, the nickel ion in filtrate was extracted and recovered with solvent of 25% N530-coal oil sulfonated. The recovery of the nickel was over 99%. The proposed method has been applied to the determination of trace nickel in red mud, the results were consistent with those obtained by catalytic spectrophotometry with the spiked recoveries of 98.3%-104.2%.
-
Keywords:
- red mud /
- nickel /
- CdTe quantum dots /
- fluorescence quenching method /
- thioglycolic acid
-
铌、钽属难熔稀有金属,它们的物理化学性质很相似,在自然界中总是相互伴生。由于铌钽矿的矿物组合复杂,干扰铌钽测定的元素种类较多,且钽、铌化学性质相似,因此铌钽矿石矿物的化学成分分析一直是研究的难点之一,尤其是测定铌、钽在百分含量以上的铌钽矿。文献中已有很多分析铌钽矿的方法,目前主要是应用电感耦合等离子体发射光谱仪(ICP-OES)[1-9]进行测定。
铌钽矿石的溶矿方法有酸溶法[1-4, 9]、微波消解法[5-6]、碱熔法[2, 7-9]等。酸溶法通常采用氢氟酸-硝酸体系消解,碱熔法通常采用过氧化钠或氢氧化钠-过氧化钠熔融。张军等[2]、许涛等[9]采用酸溶法和碱熔法处理铌钽矿样品,ICP-OES法测定,但铌钽样品铌钽含量比较低,不足0.5%,所以两种消解方法分析铌钽的结果比较一致。相比较而言,酸溶法可以提高工作效率,同时还节能环保,当样品含量低于检出限时,还可以分取稀释后用ICP-MS法测定[10-12]。但是传统的ICP-OES、ICP-MS仪器测定方法,通常需要赶掉分解的溶液中的氢氟酸,再用酒石酸保护复溶、提取,实验发现在酒石酸介质中高浓度的铌、钽的测定结果仍然会偏低,主要是因为铌、钽在蒸干形成盐类之后,很难再完全溶解到除了氢氟酸以外的溶液中,不加氢氟酸时,铌、钽部分溶解在酒石酸溶液中,形成不稳定的溶液。而经典的国家标准方法——光度法不仅测定过程繁琐,而且测定钽、铌矿石的范围比较窄(铌0.0010%~1.0%,钽0.0050%~1.0%),仅能准确测定1%以下含量的铌钽矿。
微波能穿透绝缘体介质,直接把能量辐射到有电介特性的物质上,可以完全溶解矿石中的铌、钽。但是普通的微波单次分解样品的量比较少,一般单次溶样12~24个,不能支持大批量样品的分析测试。本文采用模块化的小罐型、多罐体组合(70罐/组)的封闭性双层结构的酸溶罐体的微波消解溶样模式,样品用氢氟酸、硝酸微波消解后不需要赶氢氟酸,定容后直接利用耐氢氟酸系统的ICP-OES测定铌钽矿中铌、钽等易水解元素,建立了高、低品位铌钽矿的分析方法。
1. 实验部分
1.1 仪器及主要工作参数
Optima 8300电感耦合等离子体发射光谱仪(美国PerkinElmer公司),采用同心雾化器及旋流雾室,耐氢氟酸系统。仪器工作参数为:ICP射频功率1300 W,辅助气流量0.2 L/min,冷却气流量10.0 L/min,载气流量0.5 L/ming,氩气吹扫光路系统,轴向观测,观测距离为3,溶液提升量1.5 mL/min。
使用耐氢氟酸的刚玉中心管、雾室和雾化器。
1.2 标准溶液和主要试剂
铌、钽单元素标准储备溶液:购买浓度为1000 μg/mL(1 mol/L氢氟酸介质)单元素标准储备溶液(中国计量科学研究院)。
蒸馏水:经Mili-Q离子交换纯化系统纯化,电阻率达到18 MΩ·cm。
硝酸(1.42 g/mL),氢氟酸(1.16 g/mL)。
1.3 样品分解和测定
称取0.0500~0.1000 g(精确至0.01 mg)铌钽矿石试样(粒径应小于74 μm)放置于专用的微波消解罐中,加入1.5 mL氢氟酸和1.0 mL硝酸,密封。将消解罐放入微波消解仪中,按表 1的条件进行程序消解。冷却后取出内罐,将溶液转移至50.0 mL或100 mL塑料容量瓶中,用蒸馏水定容至刻度,此溶液直接用于ICP-OES测定(高含量的铌钽样品需要不同倍数稀释后测定)。
表 1 铌钽矿微波分解条件Table 1. The microwave decomposition conditions of niobium-tantalum ore微波消解步骤 控制温度(℃) 消解时间(min) 功率(W) 1 130 15 1200 2 160 15 1200 3 190 25 1200 点燃等离子体并稳定30 min后,用标准工作溶液对仪器进行标准化。以配制的空白溶液作为低点,用一个或多个标准溶液作为高点,以两点或多点建立校准曲线,然后对样品溶液进行测定。测定过程中,每间隔几个样品测定一个标准样品,对检测结果进行监控。
2. 结果与讨论
2.1 溶矿方式的选择
本文采用由国家地质实验测试中心与上海新仪微波仪器公司共同研制的新型微波仪器,这是一种小罐型、多罐体组合模块微波消解熔样装置。其特点是将常规的封闭溶样器和微波溶样器结合,一次可容纳70个样品溶样罐,比常用的微波溶样罐的数量(12~24个)大大增加,采用改良的聚四氟乙烯材料加工溶样内罐,比常规的封闭溶样罐[10-11]压力和温度都有一定提高(单只罐体最高耐压5 MPa,最高使用温度200℃),既提高了样品消解通量又充分利用了微波消解的能力,使其更适合于难溶地质样品的消解。
该新型微波仪器采用了航空非金属新型高强度纤维材料制作溶样罐外套,解决了常规封闭溶样器不锈钢金属外套被酸腐蚀易于污染的问题。经过多次实验,确定了微波分解铌钽矿的条件列于表 1。70个铌钽矿样品在1 h内完成了分解,而封闭酸溶[10-11]需要48 h,显著缩短了样品分解时间。而且这种新型微波仪器比普通微波仪器单次分解样品的量多出几倍,比较适合实验室的大批量样品分析,大大提高了铌钽矿的分析效率和分析结果的及时率。
2.2 耐氢氟酸进样系统测定主要原理
采用耐氢氟酸进样系统的ICP-OES和ICP-MS仪器,高、低含量的铌钽矿样品经硝酸和氢氟酸分解后不赶氢氟酸,定容后均可直接测定。邵海舟等[4]建立了用硝酸和氢氟酸溶样,使铌以稳定可溶性络合物形态存在,利用ICP-OES耐氢氟酸系统测定铌铁中的铌的含量可以达到65%。王蕾等[13]采用封闭压力酸溶的方法分解钨矿石样品,再用耐氢氟酸进样系统的ICP-OES测定钨的含量,有效地解决了钨在酸性介质中极易水解而影响其准确测定的问题。
在氢氟酸存在时,铌、钽以氟配离子状态([NbF7]2-、[TaF7]2-)存在,成为稳定的真溶液,有效防止了铌、钽的水解。

2.3 酸用量实验
由于现有的铌钽标准物质的含量比较低,实验选用铌钽含量较高的稀有稀土矿石标准物质GBW07185进行实验。称取0.1000 g的GBW07185样品9个,其中3个样品加入5.0 mL氢氟酸+1.0 mL硝酸,3个样品加入2.5 mL氢氟酸+1.0 mL硝酸,另3个样品加入1.5 mL氢氟酸+1.0 mL硝酸,微波酸溶,冷却后取出内罐,将溶液转移至50 mL塑料容量瓶中,用蒸馏水稀释定容至刻度,此溶液直接用于ICP-OES测定。测定结果(表 2)表明,三种酸溶体系都可以很好地溶解铌钽矿石,铌、钽的测定结果和推荐值一致,但考虑到氢氟酸的腐蚀性以及环境污染等因素,本方法最终选择1.5 mL氢氟酸+1.0 mL硝酸分解样品。
表 2 不同酸度条件下铌、钽测定结果Table 2. Analytical results of Nb and Ta under the different acidityGBW07185样品 5.0 mL氢氟酸+1.0 mL硝酸 2.5 mL氢氟酸+1.0 mL硝酸 1.5 mL氢氟酸+1.0 mL硝酸 Nb Ta Nb Ta Nb Ta 3次分次测定值(μg/g) 3792 3539 3701 8596 8803 8734 3733 3767 3665 8313 8518 8535 3733 3767 3665 8513 8518 8563 测定平均值(μg/g) 3677 8711 3722 8455 3722 8531 标准值(μg/g) 3635±70 8353±164 3635±70 8353±164 3635±70 8353±164 RSD(%) 3.5 1.2 1.4 1.6 1.4 0.3 相对误差(%) 1.2 4.3 2.4 1.3 2.4 2.1 2.4 称样量的影响
通过实验考察了不同称样量对分解效果的影响,分别称取50.0、100.0、200.0 mg的样品,分别加入1.5 mL氢氟酸、1.0 mL硝酸,微波消解后分别用蒸馏水定容到25、50.0、100 mL塑料容量瓶中,即稀释500倍,ICP-OES测定的结果见表 3,铌、钽含量的测定结果都与其标准值一致。
表 3 称样量的影响Table 3. Effect of sample weight称样量(mg) Nb2O5 Ta2O5 测定值 (μg/g) 标准值 (μg/g) 测定值 (μg/g) 标准值 (μg/g) 50.0 5310 10314 100.0 5281 5200±100 10457 10200±20 200.0 5258 10351 一般对粒度小于74 μm(200目)的样品测试,取样100 mg可以保证取样代表性。就仪器测定来说,称样25.00 mg就可以实现分析测定,但为了保证取样的代表性,常常要求加大取样量。从实验结果可以看出,该方法可以溶解50.0~200.0 mg的铌钽矿。
2.5 标准曲线和方法检出限
用市售的1 mg/mL铌、钽单元素标准储备溶液(1 mol/L氢氟酸介质)配制浓度为0~100 μg/mL的铌钽混合校准溶液。根据测定样品含量的高低,可以选高浓度或者低浓度系列。该方法比较常用的Nb、Ta标准系列为0.0、1.0、5.0、10.0、20.0、50.0 μg/mL,铌、钽线性方程的相关系数均大于0.9999,线性良好。
方法检出限是用该方法流程空白10次测定结果的3倍标准偏差计算,是最佳仪器条件测定。测定元素Nb所选谱线波长269.706 nm,方法检出限为5.58 μg/g;Ta所选谱线波长240.063 nm,方法检出限为5.87 μg/g。
2.6 方法精密度和准确度
选用铌、钽的标准物质GBW07154(钽矿石)、GBW07155(钽矿石),以及铌、钽含量较高的稀有稀土矿石标准物质GBW07185进行方法准确度实验。主要步骤为:称取0.1000 g的GBW07154、GBW07155、GBW07185,分别加1.50 mL 氢氟酸、1.0 mL 硝酸,按微波消解条件分解后,冷却、定容至25.0 mL,GBW07154、GBW07155溶液直接用ICP-OES测定,含量较高的GBW07185溶液稀释1倍后用ICP-OES测定。从表 4分析结果来看,测定值都与标准值相一致。
表 4 精密度和准确度实验Table 4. Precision and accuracy tests of the method标准物质编号 Nb2O5 Ta2O5 测定值(μg/g) 标准值(μg/g) RSD(%) 相对误差(%) 测定值(μg/g) 标准值(μg/g) RSD(%) 相对误差(%) GBW07154 43.3 42.3±2.5 6.0 2.3 85.9 88.6±6.0 5.2 -3.0 GBW07155 466 430±30 6.1 8.4 684 700±60 5.2 -2.3 GBW07185 5288 5200±100 3.7 1.7 10444 10200±20 1.9 2.4 由于GBW07154(钽矿石)、GBW07155(钽矿石)的铌、钽值比较低,用0.0、0.50、1.0 μg/mL三点标化的测试结果好于该方法提供的标准系列(0.0、1.0、5.0、10.0、20.0 μg/mL)。因此为了提高测定的准确度,低含量的铌、钽应该采用测定低浓度的标准系列,高浓度的溶液采用高浓度的标准系列,或者稀释后测定。实验发现,将上述的分解液稀释,采用耐氢氟酸系统的ICP-MS,能同时测定铌钽矿中的Li、W、Cu、Zn等元素。
3. 实际样品分析
由于现有的铌钽标准物质的铌、钽值较低,利用ICP-OES线性范围宽的优势,用本法测定高品位的铌钽精矿,从表 5实验结果来看,使用本方法分析铌钽精矿的测定结果与过氧化钠碱熔后ICP-OES测定的结果一致。
表 5 本法和碱熔ICP-OES方法的测定结果比较Table 5. A comparison of analytical results of Nb and Ta determined by this method and alkali fusion method样品编号 Nb2O5含量 Ta2O5含量 本方法测定值(%) 碱熔方法测定值(%) 相对误差(%) 本方法测定值(%) 碱熔方法测定值(%) 相对误差(%) 样品1 7.6 7.82 -2.7 15.32 14.7 4.2 样品2 19.28 18.99 1.5 27.4 26.9 1.9 4. 结论
本研究采用的新型微波仪器,容量大,一次可容纳70个样品溶样罐,比普通微波仪器单次分解样品量多出几倍,有利于大批量的分析测试,同时与具有耐氢氟酸系统的ICP-OES仪器相结合分析铌钽矿,得到了比较理想的实验结果,Nb2O5测定范围为42 μg/g~19%,Ta2O5测定范围为86 μg/g~27%。
该方法用硝酸和氢氟酸分解铌钽矿后不赶氢氟酸,分解液定容后直接用配制耐氢氟酸进样系统的ICP-OES测定,简化了分解流程,使得铌钽矿石中的易水解元素铌、钽的分析变得简单,提高了分析速度,尤其是解决了高品位铌钽矿的分析难题,也适合测定μg/g级低品位的铌钽原矿。
-
表 1 反应时间对CdTe量子点的荧光强度的影响
Table 1 Effects of reaction time on fluoresence intensity of CdTe QDs
反应时间
(min)荧光强度
(a.u.)反应时间
(min)荧光强度
(a.u.)1 38.4 12 79.0 5 53.9 15 78.9 10 78.6 表 2 赤泥样品中镍的分析结果
Table 2 Analytical results of nickel in red mud samples
赤泥
样品镍含量(μg/g) RSD
(%)催化光度法 本法测定值 平均值 样品1 46.61 46.70 47.15 46.32 46.55 0.8 46.11 46.38 46.64 样品2 35.92 35.73 36.47 35.74 35.97 1.5 36.08 35.16 36.64 样品3 63.26 62.45 63.36 62.73 63.22 0.8 63.41 63.86 63.48 -
-
期刊类型引用(19)
1. 李光一,马景治,李策,汪岸,贾正勋,董学林. 电弧分馏富集-发射光谱法测定含铌钽矿石中铌钽. 冶金分析. 2025(02): 49-55 .  百度学术
百度学术
2. 韩亚军,王啸,甘黎明,冯博鑫,李荣华,王佳明,宋永涛. 氟化氢铵焙烧分离-碱熔-电感耦合等离子体质谱(ICP-MS)法测定高硅矿物中稀土元素及铌、钽. 中国无机分析化学. 2025(04): 500-505 .  百度学术
百度学术
3. 洪涛,翟明国,王岳军,刘星成,徐兴旺,高俊,胡明曦,马靖. 锂铍络合物稳定性与花岗伟晶岩中锂铍“差异跃迁”耦合关联. 地学前缘. 2023(05): 93-105 .  百度学术
百度学术
4. 刘勇胜,屈文俊,漆亮,袁洪林,黄方,杨岳衡,胡兆初,朱振利,张文. 中国岩矿分析测试研究进展与展望(2011—2020). 矿物岩石地球化学通报. 2021(03): 515-539+776 .  百度学术
百度学术
5. 姚玉玲,赵朝辉,刘淑君. 树脂交换分离—电感耦合等离子质谱法测定锡矿石的铌钽. 矿产综合利用. 2021(05): 146-151 .  百度学术
百度学术
6. 郭晓瑞,樊蕾,毛香菊,张宏丽,王甜甜,姚明星. 耐氢氟酸系统进样-电感耦合等离子体质谱法测定铀铌铅多金属矿中铌. 冶金分析. 2021(11): 31-36 .  百度学术
百度学术
7. 涂建求. 电感耦合等离子体发射光谱法测定岩石矿物中的铌和钽. 江西化工. 2020(01): 73-75 .  百度学术
百度学术
8. 夏传波,成学海,赵伟,王卿,孙雨沁. 增压消解-电感耦合等离子体质谱法测定硅藻土中26种微量元素. 理化检验(化学分册). 2020(03): 277-283 .  百度学术
百度学术
9. 黄靖,王英滨,周冠轩,马真乾. 混合酸溶-电感耦合等离子体发射光谱法测定粉煤灰样品中的微量元素镓. 环境化学. 2020(05): 1427-1433 .  百度学术
百度学术
10. 秦明,朱尧伟,班俊生,杨惠玲. 微波消解–电感耦合等离子体发射光谱法测定多金属矿中10种主次元素. 化学分析计量. 2019(02): 45-49 .  百度学术
百度学术
11. 程文翠,付永立,马会春,胡艳巧,支云川,张金明,张佳林,张兆法. 纸上层析分离-ICP-AES测定稀有金属矿中的铌钽. 分析试验室. 2018(02): 168-173 .  百度学术
百度学术
12. 李刚,姚玉玲,李婧祎,赵朝辉,罗涛,李崇瑛. 铌钽元素分析技术新进展. 岩矿测试. 2018(01): 1-14 .  本站查看
本站查看
13. 邓长生,李盛富,张建梅,王明力,勒孚河,牛芳红. 常压酸溶-电感耦合等离子体质谱法测定地球化学勘查样品中的铌钽. 岩矿测试. 2018(04): 364-370 .  本站查看
本站查看
14. 李可及,赵朝辉. 低稀释比熔融-X射线荧光光谱法分析铌钽矿石. 理化检验(化学分册). 2018(12): 1410-1414 .  百度学术
百度学术
15. 吴德明,刘贝叶. 电感耦合等离子体质谱法测定铌钽矿中的铌和钽. 资源环境与工程. 2017(05): 628-630 .  百度学术
百度学术
16. 张杰芳,闫玉乐,夏承莉,焦发存,张海侠. 微波碱消解-电感耦合等离子体发射光谱法测定煤灰中的六价铬. 岩矿测试. 2017(01): 46-51 .  本站查看
本站查看
17. 王小强,夏辉,秦九红,王书勤,杨惠玲,宋志敏,杜天军. 过氧化钠碱熔-电感耦合等离子体发射光谱法测定多金属矿中的锡钨钛等主次量成分. 岩矿测试. 2017(01): 52-58 .  本站查看
本站查看
18. 宫嘉辰,姜炳南,褚晓君. 电感耦合等离子体发射光谱法测定无机纤维中的钙、镁、铝、铁. 有色矿冶. 2017(06): 53-55 .  百度学术
百度学术
19. 张纹俊,李颖. 电感耦合等离子体发射光谱法测定铌钽的研究. 低碳世界. 2016(35): 123-124 .  百度学术
百度学术
其他类型引用(2)



 下载:
下载:
 京公网安备 11010202008159号
京公网安备 11010202008159号