Selective Separation of Scandium in Acidic Water Using Carboxyl Functionalized Covalent Phosphonitrile Polymers
-
摘要:
发展高效选择性分离技术实现酸性介质中稀土元素钪(Sc)的高效提取,有利于满足工业生产过程对Sc日益增长的需求。吸附法可以有效地简化Sc的提取流程,降低生产成本。然而,现有吸附材料如硅基材料、生物质材料、金属有机骨架材料等在分离酸性介质中的Sc时仍存在效率低、选择性差等缺点,从而限制了其实际应用。因此,针对处理含Sc尾矿等原料时往往需要进行酸处理的实际情况,制备具备更强结合能力的吸附剂,实现酸性介质中Sc(III)的高效选择性分离至关重要。本文分别以间苯三酚和2,4,6-三羟基苯甲酸为构筑单元,与六氯三聚膦腈共价交联制备了共价膦腈聚合物(covalent phosphonitrile frameworks,CPF-T)和羧基功能化CPF材料(CPF-T-COOH)。采用扫描电镜、红外光谱、热重、氮气吸附-解吸等分析技术对材料的结构进行表征,并探索了它们作为Sc(III)吸附剂的应用潜力。吸附实验结果表明,在溶液pH=2时,CPF-T和CPF—COOH对Sc(III)的吸附平衡时间分别为120min和30min,最大吸附容量分别为22.48mg/g和64.63mg/g,在混合离子溶液中的分配系数分别为5.1×102mL/g和4.0×103mL/g。值得注意的是,在酸性介质(pH=1~3)中,CPF-T-COOH对Sc(III)的吸附率仍维持在95%以上,明显优于CPF-T(低于60%)以及大多数已报道的吸附材料。采用X射线光电子能谱对作用机理进一步分析,结果显示除了CPF-T骨架中N/O原子与Sc(III)的配位作用外,骨架—COOH上的O原子与-OH基团分别提供了额外的配位作用与离子交换作用,有效地增强了CPF—COOH对Sc(III)的吸附亲和力。该研究表明,利用多种功能基团的协同作用是提高吸附剂吸附性能的有效策略之一,所制备的CPF-T-COOH材料性能优异,展现了其作为Sc(III)吸附材料的良好应用前景。
Abstract:BACKGROUNDScandium (Sc) is widely used due to its excellent properties such as high melting point, high boiling point, low density, and good stability. However, as a typical dispersed element, Sc usually exists as an associated mineral. Sc needs to be recovered from the production of ore residues and tailings by-products of other metals, the production of main products from Sc containing internal (external) deposits, or from industrial wastewater and waste residue. Among numerous technologies for separating Sc, the adsorption method exhibits promising prospects due to its advantages of simple operation and high recovery. Currently, several materials including silicon-based (such as SAB-15), biomaterials, and metal organic framework, have been used for the separation of Sc(III). Although these adsorbents exhibit good adsorption ability for Sc(III), the inherent structural drawbacks such as poor chemical stability and with only one type of functional group result in the poor selectivity and inferior adsorption capacity, thereby greatly hindering their practical applications. Considering that acid treatment is often required when processing raw materials such as Sc containing tailings, it is crucial to prepare adsorbents that can efficiently and selectively recover Sc(III) in acidic media. Covalent organic polymers are porous organic polymer materials connected by covalent bonds. Due to their adjustable chemical structure, tunable pore size, and easy functionalization, they can be custom-made according to the characteristics of target ions. As one type of alternative adsorbents, they exhibit promising prospects. The pore size matching effect is conducive to achieving the efficient adsorption of target ions in acidic systems. However, because the pore size of the vast majority of covalent organic polymers currently synthesized is much larger than those of metal ions, this method is generally applicable to larger sized ions such as hydrated uranium ions. The utilization of ion imprinting technology or the ingenious selection of monomers with size matching cavities with the target ion to prepare covalent organic polymers are effective for address these issues. However, the complex preparation process makes it difficult to scale up. For covalent organic polymers, modification with special functional groups is another effective strategy to improve their adsorption performance. To tackle the issue of limited binding ability caused by the single functional group, research has shown that the introduction of various functional groups into the porous skeleton structure can effectively enhance the selective binding ability to target ions by utilizing the synergistic effect between different groups.
OBJECTIVESTo improve the adsorption performance of porous organic polymers for Sc(III) in acidic media by utilizing the synergistic effect of multiple functional groups.
METHODSPhloroglucinol (1mmol) and hexachlorocyclotriphosphazene (0.5mmol) were dissolved in 1mL of 1,4-dioxane, followed by the addition of 0.84mL of triethylamine. The mixture was transferred to a hydrothermal reactor (8mL) and reacted at 80℃ for 24h. The product was washed several times with water, ethanol and acetone, respectively. Finally, CPF-T was prepared by vacuum drying at 50℃ for 12h. CPF-T-COOH was prepared according to the same procedure by changing phloroglucinol with 2,4,6-trihydroxybenzoic acid. The infrared spectra of the CPF-T and CPF-T-COOH were collected by Thermo Scientific Nicolet iS20 Fourier transform infrared spectrometer (FT-IR) (Thermo, USA). The microstructure of the polymers was studied using SU8010 scanning electron microscope (SEM) (Hitachi, Japan). The thermogravimetric analysis (TGA) curve of the polymers was collected by the DTA7200 TGA instrument (Hitachi, Japan) under N2 conditions (N2 flow rate: 20mL/min; heating rate: 10K/min). The elemental information of the materials was analyzed using Thermo Scientific K-Alpha X-ray photoelectron spectrometer (XPS) (Thermo, USA). The N2 adsorption desorption isotherm was measured at 77K using the Micromeritics APSP2460 4-station fully automatic specific surface area analyzer (Micromeritics, USA). The sample was vacuum degassed at 120℃ for 12h before measurement. Adsorption experiments were conducted at room temperature using a constant temperature oscillator (150r/min). The adsorbent dosage was 1g/L, and the experimental data was obtained as the average of three parallel experiments. After adsorption, the supernatant was collected by filtration with 0.45μm microporous membrane. Subsequently, it was tested by EXPEC 6000 ICP-OES (Hangzhou Puyu Technology Development Co., Ltd.), and the linear correlation coefficient (R2) of the standard curve equation was >0.999.
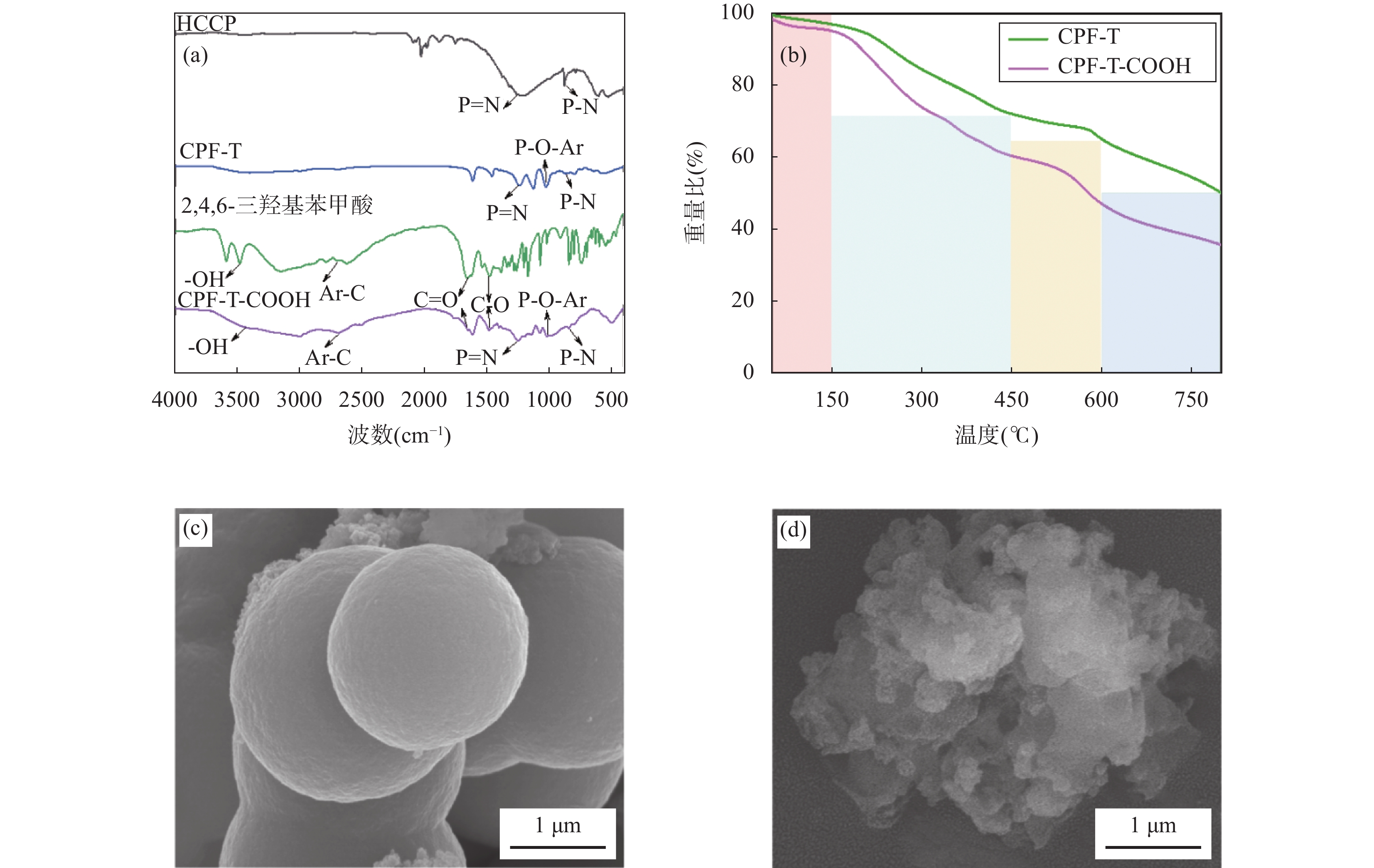
RESULTSCPF-T and CPF-T-COOH were characterized by FT-IR, SEM, TGA and N2 adsorption-desorption analysis. The appearance of P-O-Ar, P=N, and P-N stretching vibration peaks in the FT-IR diagram indicates the successful crosslinking of the organic monomers. In addition, the appearance of C=O, C-O and -OH in CPF-T-COOH indicates the successful modification of —COOH. TGA characterization indicates that the resulting materials have a good thermal stability within 150℃. Through SEM images, it can be observed that the micro morphologies of the two materials are different. CPF-T presents a relatively smooth spherical structure with some agglomerating, CPF-T-COOH exhibits an irregular, ant-like porous structure with a rough surface. The N2 adsorption-desorption isotherms of both materials belong to type II and show a strong absorption in the range of P/P0=0.8−1.0, indicating the presence of microporous and mesoporous structures. The specific surface area of CPF-T is 76.5m2/g, and total pore volume is 0.38cm3/g. The specific surface area of CPF-T-COOH is 2.61m2/g, and the total pore volume is 0.035cm3/g. The adsorption performance of CPF-T and CPF-T-COOH for Sc(III) was investigated through batch adsorption experiments, including the influence of solution pH, adsorption kinetics, adsorption isotherms and adsorption selectivity. The adsorption efficiencies of Sc(III) on the two materials exhibit different trends with respect to the solution pH. As the acidity of the solution increases, the adsorption efficiency of CPF-T for Sc(III) significantly decreases, and at pH<3, the adsorption rate is below 60%. However, within the range of solution pH=1-3, the adsorption efficiency of CPF-T-COOH for Sc(III) remains above 95%. The adsorption kinetics experiment shows that CPF-T reaches adsorption equilibrium within 120min, while CPF-T-COOH can reach adsorption equilibrium within 30min. In addition, in the adsorption isotherms experiment, the maximum adsorption capacity of CPF-T-COOH (64.6mg/g) for Sc(III) was higher than that of CPF-T (22.5mg/g). Finally, the adsorption selectivity was evaluated through coexisting ion experiments. Both materials have the ability to selectively adsorb Sc(III), with Kd values of 5.1×102mL/g and 4.0×103mL/g for Sc(III), respectively. The adsorption performance of CPF-T-COOH is not only higher than that of CPF-T, but also has unique advantages compared to some reported adsorbents, including a wider pH range, fast adsorption equilibrium time, and higher Kf value. These results indicate that the synergistic effect of multiple adsorption sites is an effective strategy for improving adsorption affinity of adsorbents. The adsorption mechanism was studied through XPS analysis. In the high-resolution XPS spectrum of N1s of Sc(III) loaded sample, all the binding energies of P-NH-P(398.0eV), P=N-H(399.3eV) and P-NH2(401.6eV) show an increase, shifting to 398.1eV, 399.6eV and 403.5eV, respectively. Meanwhile, in the spectra of O1s, it can also be observed that the binding energies of C=O(534.2eV), C-OH(533.2eV), C-O(532.0eV) and P-O(531.0eV) shift to 534.3eV, 533.3eV, 532.2eV and 531.3eV, respectively. These results indicate the existence of coordination between O/N and Sc(III). In addition, a new peak at 529.3eV corresponding to O-Sc bond appears in the spectrum of O1s for Sc(III) loading sample, and the peak area of C-OH significantly decreases. This is because the H atom in the hydroxyl group is replaced by Sc(III) through ion exchange, resulting in the formation of a new coordination bond with O.
CONCLUSIONSCovalent phosphonitrile polymer featuring abundant carboxyl functional groups is successfully synthesized by the solvothermal method. Compared with CPF-T, the carboxyl-functionalized CPF-T-COOH exhibits a much stronger binding ability toward Sc(III), where the adsorption efficiency of Sc(III) in acidic media is greatly improved from ~60% to greater than 95%. In addition, its adsorption capacity is 3.5 times that of CPF-T. The result of the mechanism study reveals that the enhanced adsorption performance is attributed to the synergistic effect of multiple functional groups, providing an alternative route for the preparation of new materials with high adsorption performance for Sc(III) capture.
-
石油作为基础性能源产品,对现代国家经济的可持续发展有着重大影响[1]。但随着石油的开发与利用,发生了一些溢油事故[2-3]给环境造成了重大危害[4]。石油类的污染物成分复杂,主要为石油烃和多环芳烃[5],其中石油烃能通过食物链富集而对人体健康造成危害[6],多环芳烃包含危害人体健康的致癌物质[7-8]。因此,对石油类污染物的监测已是环境保护的关注重点之一。2018年国家颁布的《土壤环境质量建设用地土壤污染风险管控标准(试行)》(GB 36600—2018)中将石油烃、多环芳烃等均列为监测项目,并制定了相应的风险筛选值和管控值,对土壤进行风险筛查和分类提供了依据,为生态环境修复提供了有力的技术支撑。
石油类污染物主要以烃类形式存在,碳、氢占比高达95%~99%[9],因此红外分光光度法相较于重量法[10]、紫外分光光度法[11-12]、气相色谱法[13-16]、荧光法[17-18]等,能更全面、准确地检测油类物质的总量,且灵敏度高、不受油品影响[19-20],对低含量油污染土壤测定更加适用[21-22]。石油类的官能团CH3、CH2和CH分别在红外光谱2930cm-1、2960cm-1和3030cm-1处存在伸缩振动,通过这三个波数处的吸光度可以计算出含CH3、CH2和CH基团的烃类含量[23]。现行环境标准《土壤石油类的测定红外分光光度法》(HJ 1051—2019)、《水质石油类和动植物油类的测定红外分光光度法》(HJ 637—2018)采用红外校正系数法计算石油类含量,通过测定正十六烷(CH3)、异辛烷(CH2)和苯(CH)三种烃类在三个波数下的吸光度,联立方程式计算校正因子X、Y、Z和F,利用校正因子来计算石油类含量[24],该计算方式相对复杂,手动计算费时费力;如采用软件计算虽可提高计算效率,但又因实际测试油品的红外光谱吸收峰的偏移,而造成计算结果偏差较大。在《生活饮用水标准检验方法有机物综合指标》(GB/T 5750.7—2006)、被代替的《水质石油类和动植物油的测定红外光度法》(GB/T 16488—1996)和杨斌等[25]、梁庆勋等[26]、马宏伟等[27]研究中均采用了标准曲线法,但国家标准中的标准曲线法采用非色散红外光谱单波数,因未考虑芳香烃的影响而存在局限性,从而导致标准曲线法的适用范围受限或被舍弃[28],而文献[25-27]中均未明确指出具体采用的波数,因此作为简单、方便的标准曲线法是否仍能使用,其计算结果是否具有代表性值得深究。
为解决校正系数法计算复杂、单波数计算范围受限等一系列问题,本文依据CH3、CH2和CH官能团在三个波数下产生的吸光度,组合成5种标准曲线法,计算已知含量的5种配制油品,通过计算结果的比对,确立最佳计算方法为三波数之和标准曲线法。再经过芳香烃占比试验对计算方法适用中国油品的范围进行验证。最后进行实际样品测定,并与校正系数法进行对比,验证其实用性。本文建立的三波数之和标准曲线法,为解决红外分光光度法测定石油类总量中标准曲线法的适用范围扩充提供了参考依据,同时也是对现行校正系数法的有益补充。
1. 实验部分
1.1 仪器和主要试剂
傅里叶变换红外光谱仪(FRONTIER型,美国PerkinElmer公司):扫描范围为2800~3200cm-1;配备4cm带盖石英比色皿。
四氯乙烯(红外光谱级,国药集团化学试剂有限公司)。
1.2 实验样品
标准物质:石油类标准溶液(1000mg/L)、正十六烷(10000mg/L)、异辛烷(10000mg/L)、苯(10000mg/L),均购自上海安谱实验科技股份有限公司。
其他油品:原油(华北油田);高温润滑油(长沙合轩化工科技有限公司);机油(壳牌全合成机油);0#柴油(中国石油化工集团有限公司);92#汽油(中国石油化工集团有限公司)。
实际样品:在工业园区调查项目中分别选取10个污染类型不同、污染程度不一的土壤和水质样品。土壤样品编号为T-1至T-10,水质样品编号为S-1至S-10。
1.3 标准曲线的绘制
将1000mg/L石油类标准溶液用四氯乙烯稀释成150、100、50、20、10、5、2mg/L标准系列,用4cm石英比色皿进行红外光谱扫描,记录2930cm-1、2960cm-1、3030cm-1处的吸光度值。
1.3.1 单波数标准曲线绘制
依据标准溶液浓度与2930cm-1、2960cm-1、3030cm-1处的吸光度分别绘制三条标准工作曲线。
1.3.2 两波数吸光度之和标准曲线绘制
依据标准溶液浓度与2930cm-1、2960cm-1处的吸光度之和绘制标准工作曲线。
1.3.3 三波数吸光度之和标准曲线绘制
依据标准溶液浓度与2930cm-1、2960cm-1、3030cm-1处的吸光度之和绘制标准工作曲线。
1.4 样品分析测试
1.4.1 配制油品
称取原油、润滑油、机油、柴油和汽油样品各0.50g,分别用四氯乙烯定容至50mL,配制成10000mg/L的储备液。再将上述各油品储备液用四氯乙烯稀释成100、50、20、10、5、2mg/L系列溶液,用4cm石英比色皿进行红外光谱扫描,得到红外光谱图,记录2930cm-1、2960cm-1、3030cm-1处的吸光度值。
1.4.2 配制不同浓度芳香烃的样品
以四氯乙烯为溶剂,吸取不同体积的正十六烷、异辛烷和苯标准溶液,按照不同比例配制成溶液,用4cm石英比色皿进行红外光谱扫描,使用三波数之和标准曲线法计算。
1.4.3 样品前处理和红外分光光谱分析
土壤样品:称取土壤样品10.0g于锥形瓶中,加入20mL四氯乙烯,置于振荡器中,振荡提取30min,静置10min后倾出提取液。再用20mL四氯乙烯提取一次,合并提取液并定容50mL。提取液流经填充硅酸镁吸附柱,弃去前5mL滤出液,保留剩余流出液,待测。
水质样品:取500mL水质样品于分液漏斗中,用50mL四氯乙烯分两次萃取,合并萃取液并定容至50mL。取适量萃取液过硅酸镁吸附柱,弃去前5mL滤出液,余下的接入25mL比色管中,用于测定石油类。
测定:以4cm石英比色皿加入四氯乙烯为参比,分别测量提取液的红外光谱图,记录2930cm-1、2960cm-1、3030cm-1处的吸光度值。
2. 结果与讨论
2.1 标准曲线、方法线性范围与检出限
按照1.3节标准曲线绘制步骤进行单波数、两波数吸光度之和与三波数吸光度之和绘制标准曲线,各标准曲线方程与相关系数见表 1,各浓度红外光谱图见图 1。
表 1 标准曲线方程与相关系数Table 1. Standard curve equations and correlation coefficients标准曲线名称 回归方程 相关系数(R) 2930cm-1标准曲线 y=0.0135x+0.015 0.9996 2960cm-1标准曲线 y=0.0078x-0.0041 0.9998 3030cm-1标准曲线 y=0.0011x-0.0013 0.9996 两波数吸光度之和标准曲线 y=0.0214x-0.008 0.9998 三波数吸光度之和标准曲线 y=0.0225x-0.0065 0.9999 如图 1所示,当标准溶液浓度为1mg/L时,红外吸收峰吸光度之和为0.083,虽满足3倍信噪比但峰不明显;当标准溶液浓度为150mg/L时,红外光谱图已出现平顶峰,因此石油类质量浓度在2~100mg/L时与其吸光度呈良好线性关系,相关系数如表 1所示全部大于0.999。以3倍信噪比(S/N)计算,最低检出浓度为1mg/L。
2.2 配制油品分析结果
浓度为100mg/L的5种油品的红外光谱图如图 2所示,不同产地和不同类型的油品,各种烃类的结构和所占比例相差很大,但主要属于CH2、CH3官能团组成的烷烃、环烷烃,CH官能团的芳香烃占比较少,与王玉纯等[23]采集中国不同油田的炼油厂废水进行测定得出芳香烃含量不高的结论相符。
读取上述5种油品各浓度相应波数的吸光度值,分别以2930cm-1、2960cm-1、3030cm-1的单波数标准曲线计算,以两波数吸光度之和标准曲线进行计算,以三波数吸光度之和标准曲线进行计算,得到其相应计算浓度。计算浓度(ρ计)与各油品配制浓度(ρ配)的相对误差(δ)按下列公式进行计算。
$$ \delta {\rm{ = }}\left( {{\rho _{计}} - {\rho _{配}}} \right)/{\rho _{配}} $$ 各油品单波数标准曲线计算结果的相对误差情况如图 3所示。由图 3a可知,原油和柴油的各浓度点相对误差较小,大致分布在20%之内,由此可知原油和柴油相较于其余油品更适合采用2930cm-1标准曲线进行计算。由图 3b可知,润滑油和机油的各浓度点相对误差较小,大致分布在20%之内,由此可知润滑油和机油的主要成分相较于其余油品更适合采用2960cm-1标准曲线进行计算。由图 3c可知,5种油品的各浓度点相对误差均在40%以上,计算浓度与配制浓度相差较大,表明5种油品中CH官能团为主的芳香烃含量较低或不存在[9],与图 2各油品的红外光谱图中3030cm-1峰较低或不存在的测试结果相符。单波数标准曲线的选择性强,不适用于多种类石油污染物的计算。
由图 4可知,两波数之和标准曲线法计算各油品结果的相对误差均小于30%,这是因为两波数吸光度之和标准曲线法包括了CH2、CH3两个官能团产生的吸光度(图 4a),三波数吸光度之和标准曲线法包括了CH2、CH3和CH三个官能团产生的吸光度(图 4b),较单波数标准曲线法更全面。两种方法相比较,三波数吸光度之和标准曲线法计算结果的相对误差更小,更接近于配制值,说明虽然芳香烃在石油类中含量较低,但其对总量还是存在一定的影响。所以5种标准曲线法中,三波数吸光度之和标准曲线法是更适合作为计算石油类总量的方法。
2.3 石油类污染适用范围验证结果
由2.2节可知单波数标准曲线法的选择性强,不能准确计算所有石油类污染。同样的,标准方法中的单波数非分散红外光度法由于没有考虑到芳香烃类化合物,当油品中芳烃含量超过25%时,该方法的计算结果便会产生较大误差,并不适用[28]。
为验证三波数吸光度之和标准曲线法是否存在这类问题,开展了芳香烃占比试验。表 2的计算结果表明:随着芳香烃占比的增加回收率逐渐降低,当芳香烃占比大于50%时,回收率低于70%。因为中国原油的特点是含蜡较多,属于以烷烃为主的石蜡基石油,芳香烃占比小于30%,通常油品中芳香烃含量一般不超过15%[9],所以三波数吸光度之和标准曲线法可适用于中国石油类污染的检测。
表 2 芳香烃占比试验结果Table 2. Results of the proportion test for aromatic hydrocarbons三种烃比例(正十六烷∶异辛烷∶苯) 芳香烃占比(%) 配制浓度(mg/L) 三波数之和标准曲线法 计算值(mg/L) 回收率(%) 7 ∶ 3 ∶ 0 0 50.00 59.98 119.96 6 ∶ 3 ∶ 1 10 50.00 55.42 110.83 6 ∶ 2 ∶ 2 20 50.00 52.93 105.86 5 ∶ 2 ∶ 3 30 50.00 46.70 93.39 5 ∶ 1 ∶ 4 40 50.00 43.66 87.32 3 ∶ 2 ∶ 5 50 50.00 36.55 73.10 3 ∶ 1 ∶ 6 60 50.00 33.47 66.94 2 ∶ 1 ∶ 7 70 50.00 28.24 56.48 1 ∶ 1 ∶ 8 80 50.00 22.43 44.86 1 ∶ 0 ∶ 9 90 50.00 18.65 37.29 0 ∶ 0 ∶ 10 100 50.00 13.85 27.69 2.4 方法精密度和准确度
对空白水和空白土壤(石英砂)进行加标试验,共三个浓度水平,每个浓度水平平行进行6次测定,按照1.4节进行样品前处理、三波数之和标准曲线法计算测定结果,计算其精密度与加标回收率,结果见表 3。方法精密度(RSD)在5.9%~8.0%之间,均小于10%,加标回收率在76.4%~98.2%之间,符合HJ 1051—2019、HJ 637—2018中回收率70%~110%的要求。
表 3 空白加标样品精密度结果Table 3. Precision results of blank spiked samples测定次数 土壤空白加标样品石油类物质含量(mg/kg) 水质空白加标样品石油类物质含量(mg/kg) 10mg/kg 50mg/kg 100mg/kg 0.10mg/L 0.50mg/L 2.50mg/L 1 9.11 47.6 93.4 0.0823 0.458 2.36 2 7.93 48.3 92.5 0.0764 0.403 2.13 3 9.29 47.4 91.6 0.0951 0.471 2.08 4 8.26 49.1 94.1 0.0876 0.427 2.41 5 8.74 45.7 95.7 0.0811 0.452 2.24 6 7.73 48.2 90.6 0.0798 0.485 2.33 平均值 8.51 47.7 93 0.0837 0.45 2.26 回收率(%) 77.3~92.9 91.4~98.2 91.6~95.7 76.4~95.1 80.6~97.0 83.2~96.4 RSD(%) 7.5 6.7 5.9 8.0 6.7 5.9 2.5 实际样品测定结果
按照本文的实验方法(三波数吸光度之和标准曲线法)对采集的土壤和水实际样品(1.2节)进行测定,将三波数吸光度之和标准曲线法计算结果与标准方法HJ 637—2018、HJ 1051—2019中的校正系数法计算结果进行对比。如表 4所示,对于实际土壤样品两种测试结果的相对偏差在0.5%~4.8%,水样品的相对偏差在-5.3%~6.7%,
表 4 实际样品的计算结果对比Table 4. Comparison of calculation results for actual samples土壤样品编号 土壤样品中石油类物质含量(mg/kg) 水样品编号 水样品中石油类物质含量(mg/L) 校正系数法
(标准方法)三波数之和标准曲线法
(本文方法)相对偏差
(%)校正系数法
(标准方法)三波数之和标准曲线法
(本文方法)相对偏差
(%)T-1 17.4 18.5 -3.1 S-1 0.08 0.07 6.7 T-2 9.73 9.82 -0.5 S-2 0.11 0.12 -4.3 T-3 87.9 90.9 -1.7 S-3 0.09 0.1 -5.3 T-4 104 94.8 4.6 S-4 0.67 0.65 1.5 T-5 374 393 -2.5 S-5 0.88 0.92 -2.2 T-6 646 689 -3.2 S-6 0.79 0.84 -3.1 T-7 1235 1304 -2.7 S-7 1.25 1.18 2.9 T-8 1647 1723 -2.3 S-8 1.34 1.26 3.1 T-9 5386 5839 -4.0 S-9 1.87 1.67 5.6 T-10 20880 22342 -3.4 S-10 2.07 2.14 -1.7 注:相对偏差=(推荐方法测定值-两次测定值的平均值)/两次测定值平均值×100%。 参考HJ 1051—2019中土壤平行样的相对偏差≤30%、HJ 637—2018中水样实验室内标准偏差的范围为0.8%~13%,测试结果满足要求,因此三波数吸光度之和标准曲线法可作为实际测定石油类总量的方法。
3. 结论
本文建立了三波数之和标准曲线法计算环境样品中石油类总量的方法。依据标准曲线法原理和常见油品红外谱图,对红外分光光度法测定石油类的三个波数处的吸光度进行排列组合,组建出5种标准曲线法计算已知含量的5种油品,并进行结果比对,表明三波数之和标准曲线法包含的波数全面,结果更接近实际配制值,是标准曲线法中的最佳计算方法。再经过芳香烃占比试验和实际样品验证,表明本文方法在芳香烃占比小于50%时,与校正系数法结果相一致,能满足石油类污染的测定需求。
三波数之和标准曲线法的建立,解决了标准曲线法在红外分光光度法测定石油类总量中的应用难题,突破了单波数标准曲线法的局限性,同时具有简单、方便、准确等特点,是对现行校正系数法的有益补充。但对于芳香烃占比大于50%的石油类污染,计算结果偏差较大,需进一步探讨研究。
-
图 4 溶液pH对CPF-T和CPF-T-COOH吸附Sc (III)的影响(a);吸附动力学及准一级和准二级动力学拟合曲线(b);CPF-T (c)和CPF-T-COOH (d)的吸附等温线及Langmuir和Freundlich模型拟合曲线
a—吸附剂用量1g/L,温度25℃,c0 (Sc)=50mg/L;b—pH=2,吸附剂用量1g/L,温度25℃,c0 (Sc)=50mg/L;c,d—pH=2,吸附剂用量1g/L,温度25℃。
Figure 4. The effect of solution pH on the adsorption of Sc (III) by CPF-T and CPF-T-COOH (a) (Adsorbent dosage: 1g/L; Temperature: 25℃; c0 (Sc)=50mg/L); The adsorption kinetics and fitting curves of pseudo-first-order kinetic and pseudo-second-order kinetic (b) (pH=2; Adsorbent dosage: 1g/L; Temperature: 25℃; c0 (Sc)=50mg/L); The adsorption isotherms of CPF-T (c) and CPF-T-COOH (d) and the fitting curves of the Langmuir and Freundlich models (pH=2; Adsorbent dosage: 1g/L; Temperature: 25℃).
表 1 CPF-T和CPF-T-COOH吸附Sc (III)的准一级和准二级动力学模型拟合参数
Table 1 The fitting parameters of the pseudo first-order and the pseudo second-order kinetic models for the adsorption of Sc (III) by CPF-T and CPF-T-COOH.
吸附剂 准一级动力学模型 准二级动力学模型 K1
(min−1)qe1
(mg/g)R2 K2
[g/(mg·min)]qe2
(mg/g)R2 CPF-T 0.1475 18.38 0.93 0.01195 19.83 0.98 CPF-T-COOH 0.3693 44.42 0.99 0.02216 46.78 1.0 表 2 CPF-T 和CPF-T-COOH吸附Sc(III)的Langmuir和Freundlich等温线模型拟合参数
Table 2 The fitting parameters of the Langmuir and Freundlich models for the adsorption of Sc(III) by CPF-T and CPF-T-COOH.
吸附剂 Langmuir等温线模型 Freundlich等温线模型 KL
(L/mg)qmax
(mg/g)R2 Kf
[(mg/g)·(mg/L)−1/n]n R2 CPF-T 4.099 22.48 0.93 12.21 5.998 0.74 CPF-T-COOH 5.994 64.63 0.96 49.97 15.74 0.74 表 3 CPF-T-COOH与部分已报道的Sc (III)吸附剂性能 (最佳pH、吸附平衡时间、最大吸附容量、KL值)对比
Table 3 Comparison of Sc (III) adsorption performance (the optimal pH, adsorption equilibrium time, maximum adsorption capacity, and the value of KL) between CPF-T-COOH and some reported adsorbents.
材料名称 溶液
pH吸附平衡时间
(h)最大吸附容量
(mg/g)KL
(mL/g)参考
文献0.075-AA-0.072@MIL-101 4.5 5 90.21 1.7×105 [14] BT/CoFe2O4@SiO2-CMC/PAN 5 5 49.05 2.9×105 [43] IIPBT/CoFe2O4@SiO2 5 2 128 - [51] CLx/SiO2 6 约为1 23.76 - [52] P40-750 3 约为10 18.63 3.1×105 [3] 2-MWNT-sil-P 4 24 32.92 - [53] CPF-T-COOH 2 0.5 64.63 6.0×106 本研究 注:0.075-AA-0.072@MIL-101为丙烯酸功能化的金属有机骨架材料; BT/CoFe2O4@SiO2-CMC/PAN为羧甲基壳聚糖和1-(2-吡啶偶氮)-2-萘基功能化的磁性膨润土纳米材料; IIPBT/CoFe2O4@SiO2为离子印迹纳米复合磁性膨润土; CLx/SiO2为纤维素基二氧化硅纳米复合材料; 2-MWNT-sil-P为碳纳米管和二氧化硅复合材料。 -
[1] Barteková E, Kemp R. National strategies for securing a stable supply of rare earths in different world regions[J]. Resources Policy, 2016, 49: 153−164. doi: 10.1016/j.resourpol.2016.05.003
[2] Williams-Jones A E, Vasyukova O V. The economic geology of scandium, the runt of the rare earth element litter[J]. Economic Geology, 2018, 113(4): 973−988. doi: 10.5382/econgeo.2018.4579
[3] Dai X, Thi Hong Nhung N, Hamza M F, et al. Selective adsorption and recovery of scandium from red mud leachate by using phosphoric acid pre-treated pitaya peel biochar[J]. Separation and Purification Technology, 2022, 292: 121043. doi: 10.1016/j.seppur.2022.121043
[4] Avdibegović D, Regadío M, Binnemans K. Recovery of scandium(III) from diluted aqueous solutions by a supported ionic liquid phase (SILP)[J]. RSC Advances, 2017, 7(78): 49664−49674. doi: 10.1039/C7RA07957E
[5] Yu Q, Ning S, Zhang W, et al. Recovery of scandium from sulfuric acid solution with a macro porous TRPO/SiO2-P adsorbent[J]. Hydrometallurgy, 2018, 181: 74−81. doi: 10.1016/j.hydromet.2018.07.025
[6] Lu J, Li S, Tao S, et al. Efficient CO2 electrolysis with scandium doped titanate cathode[J]. International Journal of Hydrogen Energy, 2017, 42(12): 8197−8206. doi: 10.1016/j.ijhydene.2017.01.182
[7] Zhang W, Ning S, Zhang S, et al. Synthesis of functional silica composite resin for the selective separation of zirconium from scandium[J]. Microporous and Mesoporous Materials, 2019, 288: 109602. doi: 10.1016/j.micromeso.2019.109602
[8] 杨波, 杨莉, 孟文祥. 电子探针技术探究钪在白云鄂博矿床不同矿物中的赋存特征[J]. 岩矿测试, 2022, 41(2): 185−198. doi: 10.3969/j.issn.0254-5357.2022.2.ykcs202202005 Yang B, Yang L, Meng W X. Application of electron probe microanalyzer in exploring the occurrence characteristics of scandium in different minerals of the Bayan Obo deposit[J]. Rock and Mineral Analysis, 2022, 41(2): 185−198. doi: 10.3969/j.issn.0254-5357.2022.2.ykcs202202005
[9] Hamza M F, Salih K A M, Abdel-Rahman A A H, et al. Sulfonic-functionalized algal/PEI beads for scandium, cerium and holmium sorption from aqueous solutions (synthetic and industrial samples)[J]. Chemical Engineering Journal, 2021, 403: 126399. doi: 10.1016/j.cej.2020.126399
[10] Rout P C, Sarangi K. A systematic study on extraction and separation of scandium using phosphinic acid by both solvent extraction and hollow fibre membrane[J]. Mineral Processing and Extractive Metallurgy, 2021, 131(2): 166−176.
[11] Liu C, Chen L, Chen J, et al. Application of P507 and isooctanol extraction system in recovery of scandium from simulated red mud leach solution[J]. Journal of Rare Earths, 2019, 37(9): 1002−1008. doi: 10.1016/j.jre.2018.12.004
[12] Ye Q, Li G, Deng B, et al. Solvent extraction behavior of metal ions and selective separation Sc3+ in phosphoric acid medium using P204[J]. Separation and Purification Technology, 2019, 209: 175−181. doi: 10.1016/j.seppur.2018.07.033
[13] Chen Y, Ma S, Ning S, et al. Highly efficient recovery and purification of scandium from the waste sulfuric acid solution from titanium dioxide production by solvent extraction[J]. Journal of Environmental Chemical Engineering, 2021, 9(5): 106226. doi: 10.1016/j.jece.2021.106226
[14] Lou Z N, Xiao X, Huang M N, et al. Acrylic acid-functionalized metal-organic frameworks for Sc(III) selective adsorption[J]. ACS Applied Materials & Interfaces, 2019, 11(12): 11772−11781.
[15] Zhang L, Chang X, Zhai Y, et al. Selective solid phase extraction of trace Sc(III) from environmental samples using silica gel modified with 4-(2-morinyldiazenyl)-N-(3-(trimethylsilyl)propyl)benzamide[J]. Analytical Chimica Acta, 2008, 629: 84−91. doi: 10.1016/j.aca.2008.09.039
[16] Garg R, Garg R, Okon Eddy N, et al. Biosynthesized silica-based zinc oxide nanocomposites for the sequestration of heavy metal ions from aqueous solutions[J]. Journal of King Saud University-Science, 2022, 34(4): 101996. doi: 10.1016/j.jksus.2022.101996
[17] Zhao Z, Baba Y, Yoshida W, et al. Development of novel adsorbent bearing aminocarbonylmethylglycine and its application to scandium separation[J]. Journal of Chemical Technology & Biotechnology, 2016, 91(11): 2779−2784.
[18] Zhao R, Liao H, Wu X, et al. Selective adsorption behaviours of MOFs@SiO2 with different pore sizes and shell thicknesses[J]. Journal of Solid State Chemistry, 2020, 292: 121693. doi: 10.1016/j.jssc.2020.121693
[19] Liu Z, Li H. Metallurgical process for valuable elements recovery from red mud—A review[J]. Hydrometallurgy, 2015, 155: 29−43. doi: 10.1016/j.hydromet.2015.03.018
[20] Borra C R, Blanpain B, Pontikes Y, et al. Recovery of rare earths and other valuable metals from bauxite residue (red mud): A review[J]. Journal of Sustainable Metallurgy, 2016, 2(4): 365−386. doi: 10.1007/s40831-016-0068-2
[21] Mines P D, Thirion D, Uthuppu B, et al. Covalent organic polymer functionalization of activated carbon surfaces through acyl chloride for environmental clean-up[J]. Chemical Engineering Journal, 2017, 309: 766−771. doi: 10.1016/j.cej.2016.10.085
[22] Fan L, Duan H L, Wang J, et al. Preparation of fluorinated covalent organic polymers at room temperature for removal and detection of perfluorinated compounds[J]. Journal of Hazardous Materials, 2021, 420: 126659. doi: 10.1016/j.jhazmat.2021.126659
[23] Ravi S, Kim S Y, Bae Y S. Novel benzylphosphate-based covalent porous organic polymers for the effective capture of rare earth elements from aqueous solutions[J]. Journal of Hazardous Materials, 2022, 424: 127356. doi: 10.1016/j.jhazmat.2021.127356
[24] Zhang M, Li Y, Bai C, et al. Synthesis of microporous covalent phosphazene-based frameworks for selective separation of uranium in highly acidic media based on size-matching effect[J]. ACS Applied Material & Interfaces, 2018, 10(34): 28936−28947.
[25] Li X, Qi Y, Yue G, et al. Solvent- and catalyst-free synthesis of an azine-linked covalent organic framework and the induced tautomerization in the adsorption of U(Ⅵ) and Hg(II)[J]. Green Chemistry, 2019, 21(3): 649−657. doi: 10.1039/C8GC03295E
[26] Huang J, Cui W R, Wang Y G, et al. Rational designed molecularly imprinted triazine-based porous aromatic frameworks for enhanced palladium capture via three synergistic mechanisms[J]. Chemical Engineering Journal, 2022, 430: 132962. doi: 10.1016/j.cej.2021.132962
[27] Zhang W, Wang S, Cao X, et al. A facile coordinate complexing of Na(I) to fabricate SPB-ACE@MS-4A for selective adsorption to monovalent alkali metal ions[J]. Desalination, 2021, 520: 115337. doi: 10.1016/j.desal.2021.115337
[28] Huang L J, Shen R J, Liu R Q, et al. Thiol-functionalized magnetic covalent organic frameworks by a cutting strategy for efficient removal of Hg2+ from water[J]. Journal of Hazardous Materials, 2020, 392: 122320. doi: 10.1016/j.jhazmat.2020.122320
[29] 周慧君, 帅琴, 黄云杰, 等. 双硫腙改性氧化石墨烯/壳聚糖复合微球固相萃取在线富集-原子荧光光谱法测定地质样品中痕量汞[J]. 岩矿测试, 2017, 36(5): 474−480. Zhou H J, Shuai Q, Huang Y J, et al. On-line determination of Hg(II) in geological samples by afs after solid phase extraction using dithizone-modified graphene oxide/chitosan composite microspheres[J]. Rock and Mineral Analysis, 2017, 36(5): 474−480.
[30] Ravi S, Puthiaraj P, Yu K, et al. Porous covalent organic polymers comprising a phosphite skeleton for aqueous Nd(III) capture[J]. ACS Applied Materials & Interfaces, 2019, 11(12): 11488−11497.
[31] 赵亮, 李嘉权, 王岩, 等. 氨基改性Cu-BTC对水溶液中镧铈稀土离子的吸附研究[J]. 石油化工, 2023, 52(4): 469−477. doi: 10.3969/j.issn.1000-8144.2023.04.005 Zhao L, Li J Q, Wang Y, et al. Adsorption of rare earth ions la and ce from aqueous solution by amino-modified Cu-BTC[J]. Petrochemical Technology, 2023, 52(4): 469−477. doi: 10.3969/j.issn.1000-8144.2023.04.005
[32] Xie Y, Wu Y, Liu X, et al. Rational design of cooperative chelating sites on covalent organic frameworks for highly selective uranium extraction from seawater[J]. Cell Reports Physical Science, 2023, 4(1): 101220. doi: 10.1016/j.xcrp.2022.101220
[33] Liu M, Chen J, Zou D, et al. A novel synergistic extraction system for the recovery of scandium(III) from sulfuric acid medium with mixed cyanex923 and N1923[J]. Separation and Purification Technology, 2022, 283: 120223. doi: 10.1016/j.seppur.2021.120223
[34] Hu Z P, Jiang H L, Hu Q D, et al. Preparation of the hexachlorocyclotriphosphazene crosslinked sodium alginate polymer/multi-walled carbon nanotubes composite powder for the removal of the cationic dyes[J]. Journal of Molecular Structure, 2022, 1262: 133050. doi: 10.1016/j.molstruc.2022.133050
[35] Lv M, Yao C, Yang D, et al. Synthesis of a melamine-cyclotriphosphazene derivative and its application as flame retardant on cotton gauze[J]. Journal of Applied Polymer Science, 2016, 133(25): 43555.
[36] Mohamed M G, Hsiao C H, Luo F, et al. Multifunctional polybenzoxazine nanocomposites containing photoresponsive azobenzene units, catalytic carboxylic acid groups, and pyrene units capable of dispersing carbon nanotubes[J]. RSC Advances, 2015, 5(56): 45201−45212. doi: 10.1039/C5RA07983G
[37] Liu R, Yan Q, Tang Y, et al. Nacl Template-assisted synthesis of self-floating COFs foams for the efficient removal of sulfamerazine[J]. Journal of Hazardous Materials, 2022, 421: 126702. doi: 10.1016/j.jhazmat.2021.126702
[38] Xu S, Weng Z, Tan J, et al. Hierarchically structured porous organic polymer microspheres with built-in Fe3O4 supraparticles: Construction of dual-level pores for Pt-catalyzed enantioselective hydrogenation[J]. Polymer Chemistry, 2015, 6(15): 2892−2899. doi: 10.1039/C4PY01611D
[39] Ma Z, Liu F, Liu N, et al. Facile synthesis of sulfhydryl modified covalent organic frameworks for high efficient Hg(II) removal from water[J]. Journal of Hazardous Materials, 2021, 405: 124190. doi: 10.1016/j.jhazmat.2020.124190
[40] Chen Y, Wang S, Li Y, et al. Adsorption of Pb(II) by tourmaline-montmorillonite composite in aqueous phase[J]. Journal of Colloid and Interface Science, 2020, 575: 367−376. doi: 10.1016/j.jcis.2020.04.110
[41] Wang P, Ding F, Huang Z, et al. Adsorption behavior and mechanism of Cd(II) by modified coal-based humin[J]. Environmental Technology & Innovation, 2021, 23: 101699.
[42] Wood S A, Samson I M. The aqueous geochemistry of gallium, germanium, indium and scandium[J]. Ore Geology Reviews, 2006, 28(1): 57−102. doi: 10.1016/j.oregeorev.2003.06.002
[43] Zhang L, Wang C, Yang R, et al. Novel environment-friendly magnetic bentonite nanomaterials functionalized by carboxymethyl chitosan and 1-(2-pyridinylazo)-2-naphthaleno for adsorption of Sc(III)[J]. Applied Surface Science, 2021, 566: 150644. doi: 10.1016/j.apsusc.2021.150644
[44] Salman A D, Juzsakova T, Akos R, et al. Synthesis and surface modification of magnetic Fe3O4@SiO2 core-shell nanoparticles and its application in uptake of scandium(III) ions from aqueous media[J]. Environmental Science Pollution Research, 2021, 28(22): 28428−28443. doi: 10.1007/s11356-020-12170-4
[45] Wang J, Guo X. Adsorption kinetic models: Physical meanings, applications, and solving methods[J]. Journal of Hazardous Materials, 2020, 390: 122156. doi: 10.1016/j.jhazmat.2020.122156
[46] Xu W, Zhou S, Wang B, et al. Efficient adsorption of Au(III) from acidic solution by a novel N, S-containing metal–organic framework[J]. Separation and Purification Technology, 2022, 288: 120646. doi: 10.1016/j.seppur.2022.120646
[47] 杨梦楠, 孙晗, 曹海龙, 等. 生物炭-壳聚糖磁性复合吸附的制备及去除地下水中铅和铜[J]. 岩矿测试, 2023, 42(3): 563−575. Yang M N, Sun H, Cao H L, et al. Preparation and application of biochar-chitosan magnetic composite adsorbent for removal of lead and copper from groundwater[J]. Rock and Mineral Analysis, 2023, 42(3): 563−575.
[48] Wang J, Guo X. Adsorption isotherm models: Classification, physical meaning, application and solving method[J]. Chemosphere, 2020, 258: 127279. doi: 10.1016/j.chemosphere.2020.127279
[49] Li X, Liu Z, Wei L, et al. Comparison of competitive and synergistic adsorption of tetrabromobisphenol-A and its metabolites on two different organic-modified clays[J]. Journal of Chemical & Engineering Data, 2019, 64(6): 2780−2790.
[50] Tran H N, Bonilla-Petriciolet A. Improper estimation of thermodynamic parameters in adsorption studies with distribution coefficient Kd (Qe/Ce) or Freundlich constant ( Kf): Considerations from the derivation of dimensionless thermodynamic equilibrium constant and suggestions[J]. Adsorption Science & Technology, 2022, 2022: 1−23.
[51] Wang C, Zhang L, Zhou G, et al. Synthesis of environmental-friendly ion-imprinted magnetic nanocomposite bentonite for selective recovery of aqueous Sc(III)[J]. Journal of Colloid and Interface Science, 2023, 630: 738−750. doi: 10.1016/j.jcis.2022.10.161
[52] Iftekhar S, Srivastava V, Sillanpää M. Enrichment of lanthanides in aqueous system by cellulose based silica nanocomposite[J]. Chemical Engineering Journal, 2017, 320: 151−159. doi: 10.1016/j.cej.2017.03.051
[53] Ramasamy D L, Puhakka V, Doshi B, et al. Fabrication of carbon nanotubes reinforced silica composites with improved rare earth elements adsorption performance[J]. Chemical Engineering Journal, 2019, 365: 291−304. doi: 10.1016/j.cej.2019.02.057
[54] Wu J S, Li Z, Tan H X, et al. Highly selective separation of rare earth elements by Zn-Btc metal-organic framework/nanoporous graphene via in situ green synthesis[J]. Analytical Chemistry, 2021, 93(3): 1732−1739. doi: 10.1021/acs.analchem.0c04407
[55] 王哲, 朱俊, 李雯, 等. 镧沸石对磷和重金属的吸附与底泥钝化性能[J]. 环境科学, 2022, 43(11): 5106−5114. Wang Z, Zhu J, Li W, et al. Adsorption of phosphate and heavy metals by lanthanum modified zeolite and its performance in sediment inactivation[J]. Environmental Science, 2022, 43(11): 5106−5114.
[56] He J, Lu Y, Luo G. Ca(II) imprinted chitosan microspheres: An effective and green adsorbent for the removal of Cu(II), Cd(II) and Pb(II) from aqueous solutions[J]. Chemical Engineering Journal, 2014, 244: 202−208. doi: 10.1016/j.cej.2014.01.096
[57] Gao X, Zhang Y, Zhao Y. Biosorption and reduction of Au(III) to gold nanoparticles by thiourea modified alginate[J]. Carbohydrate Polymers, 2017, 159: 108−115. doi: 10.1016/j.carbpol.2016.11.095
-
期刊类型引用(6)
1. 卢碧翠,张修华,王智青,张红进,刘丽立,潘晓瑜,杨明生,黄中伟,陈奇志. 提高电解二氧化锰中间控制磨粉样铁含量检测效率的研究. 中国锰业. 2024(03): 60-63 .  百度学术
百度学术
2. 杨精存,丁杭冰,施亚菁. 磁性固相萃取-电感耦合等离子体质谱(ICP-MS)法同时检测制革废水多种重金属元素. 皮革与化工. 2024(05): 20-25 .  百度学术
百度学术
3. 冯先进,杨斐. 电感耦合等离子体串联质谱技术特点及国内应用现状. 冶金分析. 2023(09): 1-13 .  百度学术
百度学术
4. 严煜,韩乃旭,卢水淼,夏晓峰,林黎,张秀丽. 工业在线-电感耦合等离子体发射光谱法分析湿法冶炼硫酸锌溶液中铜镉钴铁. 岩矿测试. 2022(01): 153-159 .  本站查看
本站查看
5. 王干珍,彭君,李力,秦毅,曹健,田宗平. 锰矿石成分分析标准物质研制. 岩矿测试. 2022(02): 314-323 .  本站查看
本站查看
6. 王凯凯. 等离子质谱在水环境重金属检测中的应用. 冶金管理. 2021(11): 163-164 .  百度学术
百度学术
其他类型引用(0)



 下载:
下载:









 京公网安备 11010202008159号
京公网安备 11010202008159号