Abnormal Hot Blank of Oxygen-free Copper Sample Holder and Implications for Laser 40Ar/39 Ar Dating
-
摘要:
激光40Ar/39Ar定年方法中扣除的本底是样品测试过程中的系统冷本底。在满足激光加热样品时样品盘升温幅度有限和样品盘已完全脱气两个条件的情况下,这一处理方式的有效性才能得到保证。本文利用具有不同大气暴露史的无氧铜样品盘结合透长石标准样品YBCs,使用相同的脱气及测试流程,对比分析了不同样品盘的冷、热本底以及放置于不同样品盘时YBCs的大气氩含量。分析结果表明,放置于暴露大气14个月的样品盘内时,YBCs透长石大气氩含量高达约34.4%,使用预先激光去气的样品盘此值可降低至约2%;暴露大气约10个月的样品盘,激光加热其两个样品孔时,40Ar脱气量可达约1.6×10−14~3.1×10−14mol;暴露时长约为26个月的样品盘,40Ar含量升高至约0.8×10−13~2.0×10−13mol;它们均远高于系统冷本底3.8×10−16~6.2×10−16mol。两个样品盘热本底40Ar/36Ar值约为310,高于大气氩比值。因此,对于暴露大气时间较长的样品盘,约150℃去气四天的流程不足以使其完全脱气。激光加热样品时会导致样品盘局部升温,脱气不完全的样品盘会释放出大量热本底。模拟以及标准样品测试均显示了这种情况会影响辐照参数J值以及年龄的计算。激光微量年轻样品40Ar/39Ar定年过程中,建议装样后对无氧铜样品盘进行300~400℃至少5h的预脱气,以保证测试数据质量。实验室不具备预脱气条件时,持续使用同一样品盘也可以有效地降低异常热本底对测试结果的影响。
-
关键词:
- 激光40Ar/39Ar定年 /
- 无氧铜样品盘 /
- 热本底 /
- 脱气 /
- 质谱分析
Abstract:BACKGROUNDOxygen-free copper (OFC) tray is usually used as a container for samples of laser 40Ar/39Ar dating. The blank of the mass spectrometry system needs to be subtracted from the sample signal before age calculation. The tray’s hot blank of argon, including its amount and isotope ratio will affect the 40Ar/39Ar age calculation. However, the material and structure of the laser window make the laser chamber susceptible to temperatures higher than 150°C during the degassing procedure of laser 40Ar/39Ar dating[4,10]. This temperature may be too low to degas the OFC tray completely during the experiment. Besides, the wells loading the mineral samples limit the direct acquisition of the hot blank of the system. These two points make it very difficult to accurately deduct the blank signal of laser 40Ar/39Ar dating.
OBJECTIVESTo testify to the effectiveness of the traditional degassing procedure on the sample holder and evaluate the effect of hot blank on age calculation of laser 40Ar/39Ar dating.
METHODSTo confirm the effectiveness of the degassing procedure, the hot blank of OFC trays, which had been exposed to atmosphere for different time intervals were measured by mass spectrometry, after degassing the laser chamber to 150℃ for four days. Loading the standard mineral YBCs sanidine in the well in OFC trays with different duration in air, the argon isotopes were measured after the same degassing procedure. The change of the proportion of atmospheric argon of YBCs will verify if laser energy heats the OFC tray and mineral sample simultaneously.
RESULTSThe temperature of the sample holder increases during laser heating of samples. For standard mineral YBCs sanidine placing in OFC tray C, exposed to the atmosphere for about 14 months, the proportion of atmospheric argon was 34.4% (Table 1). This value decreased to 2.0% when YBCs was placed in tray D and degassed by laser at higher energy. This implies that the tray temperature will increase during sample heating by laser, and a considerable amount of gas will be released if the tray is not completely degassed. It was proved that heated to about 150℃ was not enough to completely degas the OFC tray during experiment. The hot blank of another two planchettes following the same degassing procedure were measured. For tray A with exposure to the atmosphere for 10 months, the amount of 40Ar released from two wells reached 1.6×10−14−3.1×10−14mol; for tray B with exposure to the atmosphere for 26 months, the 40Ar content increased to 0.8×10−13−2.0×10−13mol. These levels were much higher than the cold background of 3.8×10−16−6.2×10−16mol. Incomplete degassing of the tray may lead to argon isotopic fractionation, resulting in the value of 40Ar/36Ar of the hot blank rise to 310, which is higher than the value of atmospheric argon. Under this condition, using 40Ar/36Ar=295.5[16] or 298.56[17] to correct the atmospheric argon will add extra 40Ar to the sample’s signal and lead to an older age. The OFC tray was degassed efficiently while heated with a higher laser power. Under the same degassing procedure, the hot blank of the empty wells in tray D was similar to the cold blank of the system. The OFC trays exposed to air also gave the same conclusion. When the wells in the tray were heated by a lower power energy following a higher power energy, the hot blank dropped to the cold blank level (Fig.2). Assuming all of the atmospheric argon contributed from the hot blank with 40Ar/36Ar=310, its effect on 40Ar/39Ar age calculation with different combination of 40Ar*/39ArK, J-value and atmospheric argon content was calculated. Results show that the percent change of age(t%) was mainly controlled by the atmospheric argon content. When the atmospheric argon content increased to 50% (Fig.3), it elevated the age by 5%. Under the same condition, the J-value was about 5.2% lower(Fig.4). In practice, however, the different capacity of adsorption and thermal desorption of gas of different minerals[18-19] makes the effects difficult to quantify.
CONCLUSIONSCareful degassing of the OFC tray is needed, especially in very young samples dating through the laser heating method. The temperature of the sample holder increases when the sample is heated by laser, releasing considerable amounts of gas if the holder is incompletely degassed. The limited temperature of the degassing procedure cannot degas the sample holder completely, if the holder adsorbs a lot of gases. There are two possible solutions to solve this problem. The first is pre-degassing the sample holder at 300-400℃ for at least 5 hours at high vacuum furnace after sample exchange. The second is reusing the same sample holder continuously in laser 40Ar/39Ar dating. A combination of both solutions is likely to be most effective.
-
电解二氧化锰主要为γ-MnO2结构,较大的尺寸隧道有利于离子扩散,放电过程中极化小,具有电化学活性强、纯度高、成本低等优势,在电化学储能领域一直占据着至关重要的核心地位[1-3]。电解二氧化锰废渣是在电解二氧化锰的生产过程中,锰矿经硫酸浸出后压滤固液分离产生的废渣,具有成分复杂、形态性质多变、有毒有害、不易降解等特点[4]。在长期堆放过程中不仅会占据大量的土地资源,而且破坏生态环境。因此,对于电解二氧化锰废渣的处理与安全监测显得尤为重要,国家标准《危险废物鉴别标准通则》(GB 5085.7—2019)对固体废弃物中的重金属元素浸出量制定了严格的限量标准:Cr<5mg/L,Ni<5mg/L,As<5mg/L,Cd<1mg/L,Hg<0.11mg/L,Pb<5mg/L。
在大气降雨的作用下,电解二氧化锰废渣中的可溶性重金属进入土壤和水体,从而对下游水生态系统及农业生生态系统造成不同程度的环境污染和安全隐患[5-8],尤其是重金属元素通过食物链进入人体后,其毒性放大[9-11],并能与水中其他毒素结合生成毒性更大的化合物,从而对人体健康造成更大危害[12],开展电解二氧化锰废渣浸出液中重金属元素的测定,对于人们更加深入地了解电解二氧化锰废渣浸出液中重金属元素在环境中的分布状态和迁移过程具有十分重要的意义。
目前,有关重金属元素的含量主要采用原子光谱法进行测定[13-16],而对于锰废渣浸出液中重金属元素的分析报道不多。胡南等[17]采用原子吸收光谱法(AAS)测定了硫酸锰废渣浸出液中重金属元素Pb、Zn、Mn、Hg、Cu、Cd、As,均高于《污水综合排放标准》(GB 8978—1996)规定的限量标准,但AAS法原子化温度低,对难电离元素以及非金属元素的检测能力较差,且不能同时进行多元素的测定;周亚武等[18]采用电感耦合等离子体发射光谱法(ICP-OES)对锰渣浸出液中重金属元素Mn、Cr、Pb、Zn、Cd进行了测定,表明锰渣浸出液中的主要污染物为Mn、Cr两种重金属,受检出能力的限制,ICP-OES不能进行痕量和超痕量元素的测定;罗乐等[19]应用电感耦合等离子体质谱法(ICP-MS)研究了锰渣浸出液中重金属元素As、Cd、Cr、Cu、Pb、Zn的最佳浸出条件,在酸性环境下,浸出率随酸性的增强逐渐升高。然而,ICP-MS法对于存在严重干扰元素(如Cr、Ni、As)的测定表现为灵敏度低和检出限差,尤其是电解二氧化锰废渣浸出液中重金属元素的含量通常很低,使用四极杆ICP-MS(ICP-QMS)很难实现这些元素的准确测定。碰撞反应池(CRC)为ICP-QMS消除质谱干扰提供了通用技术[20-22],但基于动能歧视(KED)的氦碰撞模式下仅能消除多原子离子干扰,而不可预知的反应历程和反应产物制约了反应模式的潜能[23]。电感耦合等离子体串联质谱(ICP-MS/MS)是在带CRC的ICP-QMS基础上新增一级四极杆质量过滤器(Q1),形成串联质谱,通过Q1阻止大量干扰离子进入CRC,严格控制CRC内的碰撞/反应过程和产物离子,提高了分析元素的检测能力[24-27],已广泛应用于复杂样品基质中痕量元素的分析[28-30],但目前有关ICP-MS/MS测定电解二氧化锰废渣浸出液中重金属元素的分析方法有待探索。
本文采用ICP-MS/MS直接测定电解二氧化锰废渣浸出液中6个重金属元素Cr、Ni、As、Cd、Hg、Pb,依据《固体废物浸出毒性浸出方法硫酸硝酸法》(HJ/T 299—2007)对电解二氧化锰废渣中的重金属元素进行浸出。针对分析Cr、Ni、As、Cd存在的严重质谱干扰,通过优化ICP-MS/MS工作条件,在MS/MS模式下应用O2为反应气,使Cr+、Ni+和As+与O2反应分别生成CrO+、NiO+和AsO+进行测定,利用质谱转移法消除干扰;使干扰Cd+测定的离子与O2反应发生质量转移,而Cd+不与O2反应,利用原位质量法消除干扰,以期为电解二氧化锰废渣浸出液中重金属元素的快速准确、测定提供一种高通量分析方法。
1. 实验部分
1.1 仪器及工作条件
Agilent 8800型电感耦合等离子体串联质谱仪(美国Agilent公司)。优化后ICP-MS/MS的工作条件为:预设等离子体,耐高盐的进样系统HMI参数设置为“中等”;射频功率1550W;扫描模式MS/MS;采样深度8mm;载气流速0.70L/min;补偿气流速0.50L/min;池反应气(O2)流速0.5mL/min;八极杆偏置电压-14V;动能歧视电压-8V;所选同位素:52Cr、60Ni、75As、111Cd、202Hg、208Pb。
Milli-Q超纯水机(美国Millipore公司)。
1.2 标准溶液和主要试剂
1000mg/L单元素标准储备溶液(国药集团化学试剂有限公司);1000mg/L的Sc、Y、Bi内标单元素标准储备溶液(国药集团化学试剂有限公司)。
优级纯硝酸和优级纯硫酸(德国Merck公司)。
1.3 实验样品
标准物质粉煤灰(SRM 1633c)来自于美国国家标准及技术研究所(NIST),用于验证分析方法的准确性。
为减少堆放环境对电解二氧化锰废渣浸出液的影响,实验样品选取4批新鲜电解二氧化锰废渣(样品编号A、B、C、D)。其呈黑色泥糊状,由湖南湘潭市华昇环保科技有限公司提供,烘干除去水分后按实验方法浸出重金属元素。
1.4 实验方法
按《固体废物浸出毒性浸出方法硫酸-硝酸法》(HJ/T 299—2007)对已烘干的电解二氧化锰废渣样品进行破碎、过9.5mm筛网处理。准确称取电解二氧化锰废渣100g于2000mL聚乙烯(PE)提取瓶中,加入1000mL浸取剂(取质量比为2:1的浓硫酸和浓硝酸混合液约2滴加入到超纯水中,使溶液的pH为3.20±0.05),翻转式振荡18h浸出重金属元素,过滤得到浸出液后直接采用ICP-MS/MS进行测定。所有测定溶液(样品溶液、标准溶液、空白溶液)均采用“T”形内标混合接头在线加入1mg/L的Sc、Y、Rh、Bi内标溶液,实验数据采用Agilent MassHunter软件进行处理。
2. 结果与讨论
2.1 质谱模式的选择
电解二氧化锰废渣的基质组成复杂。所形成的质谱干扰不仅会严重干扰轻质量同位素52Cr、60Ni、75As(质荷比m/z<80的同位素)的测定,还会对中质量同位素111Cd形成干扰(表 1),而对重质量同位素202Hg和208Pb的干扰可以忽略不计。为获得分析元素的最佳质谱工作模式,在三种质谱模式下进行了数据采集:单四极杆(SQ)无气体模式、SQ碰撞模式和MS/MS反应模式。通过对比不同质谱模式下各元素背景等效浓度(BEC)的变化来考察质谱干扰的消除效果。
从表 1可以看出,在SQ模式下,采用无气体方式测定同位素52Cr、60Ni、75As、111Cd,由于没有消除干扰,4个同位素的BEC值均处于较高水平,其中52Cr的干扰最严重,BEC最大;在SQ模式下,采用He为碰撞气,由于分析元素的质谱干扰主要为多原子离子干扰,52Cr、60Ni、75As、111Cd的BEC值变小,表明消除了大部分干扰,而202Hg和208Pb在He碰撞模式下BEC值反而变大,表明Hg和Pb几乎没有质谱干扰,碰撞过程中由于能量的损失导致Hg和Pb的BEC值反而变大。
表 1 不同质谱模式下分析元素的背景等效浓度Table 1. Background equivalent concentrations of analytes in different mass spectrometric mode同位素 潜在质谱干扰 背景等效浓度(ng/L) SQ(无气体模式) SQ(He碰撞模式) MS/MS(O2反应模式) 52Cr 40Ar12C, 35Cl16O1H, 36Ar16O, 38Ar14N 31600 53.7 23.4 60Ni 59Co1H, 23Na36Ar1H, 23Na37Cl 78.9 40.5 5.28 75As 40Ar35Cl, 59Co16O, 150Nd++, 150Sm++ 1050 22.8 13.0 111Cd 95Mo16O 216 84.6 19.2 202Hg 186W16O 10.3 17.1 16.6 208Pb 192Os16O 11.2 15.8 20.5 在MS/MS模式下,对于52Cr的测定,设置Q1的m/z为52,将大量m/z≠52的干扰离子排除在外,仅允许m/z=52的离子进入CRC内,采用O2为反应气,52Cr+与O2发生反应生成52Cr16O+,而52Cr+的干扰离子均不与O2发生反应[31],设置二级四极杆质量过滤器(Q2)的m/z=68,利用质量转移法消除52Cr的所有质谱干扰。60Ni+在O2反应模式下的质谱行为与52Cr+相似,而75As+与O2反应能自发进行[32-34],60Ni+和75As+均采用O2质量转移法消除干扰。111Cd+不与O2发生质量转移反应,而111Cd+的干扰离子95Mo16O+能与O2发生二次质量转移反应生成95Mo16O2+[35],设置Q1和Q2的m/z均为111,利用原位质量法消除干扰,从而实现了111Cd的无干扰测定。从表 1可以看出,在MS/MS模式下,52Cr、60Ni、75As、111Cd的BEC值明显低于SQ模式。本实验消除质谱干扰的原理见图 1。
同位素202Hg和208Pb受到的质谱干扰轻微,可能忽略不计,在MS/MS模式下的BEC值反而高于SQ无气体模式,表明分析离子经过Q1和Q2过滤后有能量损失,对于Hg和Pb的测定,本实验采用SQ无气体模式进行测定。
2.2 反应气O2流速的优化
反应气O2流速决定CRC内O2浓度,影响质谱干扰的消除效果和分析元素的信号强度[24]。过低的O2流速会导致反应不完全,生成的产物离子浓度低,对于采用质量转移法消除干扰的分析元素表现为信号强度弱,灵敏度低,对于采用原位质量法消除干扰的分析元素则表现为消除干扰不彻底;而过高的O2流速会增加分析元素与O2碰撞次数,导致分析元素信号强度降低[36]。本实验针对52Cr、60Ni、75As、111Cd的潜在质谱干扰,采用模拟干扰溶液来优化O2流速,配制浓度为10μg/L的Cr、Ni、As、Cd分析元素和浓度为1g/L的C、Cl、Co、Na、Mo干扰元素组成的混合标准溶液,考察不同O2流速下质谱干扰的消除效果,结果如图 2所示。随着O2流速的增大,Cr、Ni、As、Cd的测定值逐渐接近标准值10μg/L,当O2流速达到0.4mL/min后,这4个元素的测定值与标准值一致,表明消除了所有质谱干扰。为确保所有反应彻底进行,本实验最终选择O2流速为0.5mL/min。
2.3 内标元素的分配与方法检出限
锰渣样品浸出中的复杂基质产生基体效应,选择加入内标元素既能校正基体效应,也能防止分析元素的质谱信号出现漂移[37-39]。然而,在MS/MS反应模式下,由于内标元素可能与O2反应发生质量转移,使内标元素的选择变得更加复杂。本实验选择在线加入1mg/L的Sc、Y、Rh、Bi混合内标元素,其中,45Sc+与O2发生反应为放热过程,能够自发生成丰度高且无干扰的45Sc16O+,用作52Cr16O、60Ni16O的内标元素;同理,89Y+与O2发生反应也是放热过程,自发生成大量的89Y16O+用作75As16O的内标元素;而103Rh+几乎不与O2发生反应,适合用作111Cd的内标元素;在Hg和Pb的测定过程中,没有使用CRC,因此实验选择209Bi用作Hg和Pb的内标元素。
分别配制0.0、0.5~5.0μg/L(0.5μg/L的Cd、Hg,5.0μg/L的Cr、Ni、As、Pb),2.0~20μg/L(2.0μg/L的Cd、Hg,20μg/L的Cr、Ni、As、Pb),10~100μg/L(10μg/L的Cd、Hg,100μg/L的Cr、Ni、As、Pb),50~500μg/L(50μg/L的Cd、Hg,500μg/L的Cr、Ni、As、Pb)系列混合标准溶液,在优化条件下进行测定,得到待测元素的校准数据。通过表 2中数据可以看出,6个待测元素在各自线性范围内线性相关系数≥0.9998,线性关系良好,Cr、Ni、As、Cd、Hg、Pb元素的仪器检出限分别为3.06、9.31、3.50、2.72、2.03、1.89ng/L。
表 2 校准数据与检出限(n=11) Table 2. Calibration data and detection limits(n=11) 待测
元素监测离子 内标分配 线性范围
(μg/L)线性相
关系数检出限
(ng/L)Cr 52Cr16O+ 45Sc16O+ 10.2~500 1.0000 3.06 Ni 60Ni16O+ 45Sc16O+ 31.3~500 0.9999 9.31 As 75As16O+ 89Y16O+ 11.7~500 0.9998 3.50 Cd 111Cd+ 103Rh+ 9.07~50 1.0000 2.72 Hg 202Hg+ 209Bi+ 6.77~50 0.9999 2.03 Pb 208Pb+ 209Bi+ 6.30~500 1.0000 1.89 2.4 方法准确度和精密度的验证
由于缺少电解二氧化锰废渣标准参考物质,采用三水平加标回收实验验证方法的准确度和精密度,每个样品重复测定6次,计算加标回收率和相对标准偏差(RSD),分析结果见表 3。样品各元素的加标回收率在93.7%~107.2%之间,RSD≤3.9%,表明所建立的分析方法准确度高,精密度好。
表 3 分析方法的准确度和精密度(n=6) Table 3. Accuracy and precision of analytical method(n=6) 待测元素 加标值
(μg/L)测定值
(μg/L)加标回收率
(%)RSD
(%)2.00 1.88 94.0 2.8 Cr 10.0 10.3 103.0 2.2 50.0 51.6 103.2 1.9 2.00 2.05 102.5 2.6 Ni 10.0 9.87 98.7 3.0 50.0 51.8 103.6 2.5 2.00 1.95 97.5 1.7 As 10.0 10.4 104.0 3.1 50.0 52.3 104.6 2.0 2.00 1.92 96.0 2.3 Cd 10.0 9.37 93.7 3.9 50.0 53.6 107.2 3.2 2.00 1.90 95.0 2.7 Hg 10.0 9.62 96.2 3.4 50.0 47.3 94.6 2.5 2.00 2.07 103.5 1.8 Pb 10.0 10.1 101.0 2.1 50.0 48.4 96.8 2.9 2.5 样品分析
采用建立的ICP-MS/MS方法分别对4个电解二氧化锰废渣浸出液中的6个重金属元素进行测定,所有电解二氧化锰废渣浸出液样品均为淡蓝色澄清溶液,每个样品重复测定6次,分析结果见表 4。电解二氧化锰废渣浸出液中Cr含量最高,Ni、As、Cd、Hg、Pb含量均处于较低水平,其中Hg含量最低。电解二氧化锰废渣浸出的6个重金属元素浓度均低于GB 5085.7—2019中的限值标准。
表 4 电解二氧化锰废渣浸出液的分析结果(n=6) Table 4. Analytical results of leaching solution of electrolytic manganese dioxide waste residue(n=6) 待测
元素元素测定值(μg/L) 样品A 样品B 样品C 样品D Cr 46.2±1.70 32.8±1.26 61.3±2.19 13.4±0.51 Ni 0.71±0.028 0.92±0.037 0.38±0.014 0.25±0.010 As 3.06±0.12 1.37±0.048 0.88±0.035 5.41±0.16 Cd 0.072±0.005 0.040±0.003 0.052±0.003 0.087±0.005 Hg 0.053±0.004 0.031±0.002 0.016±0.001 0.023±0.002 Pb 0.38±0.011 0.69±0.025 0.45±0.018 0.82±0.034 3. 结论
建立了采用ICP-MS/MS测定电解二氧化锰废渣浸出液中6个重金属元素的分析方法。通过在MS/MS模式下采用O2为反应气,与传统的ICP-QMS以及配有碰撞反应池(CRC)的ICP-MS相比,消除干扰更加彻底,待测元素的检出限均达到ng/L级水平。本方法具有分析速度快、准确度高、精密度好的优势,能满足电解二氧化锰废渣浸出液中重金属元素的测定要求。
本研究已应用于湖南湘潭、花垣以及重庆秀山大量电解二氧化锰废渣浸出液中重金属元素的环境评价项目,同时也可应用于其他工业废渣浸出液中重金属元素的测定,显示出高通量分析特性。本方法通过后续实验条件的优化,可同时分析废渣浸出液中难电离非金属元素如磷、硫、硅、氯的测定。
-
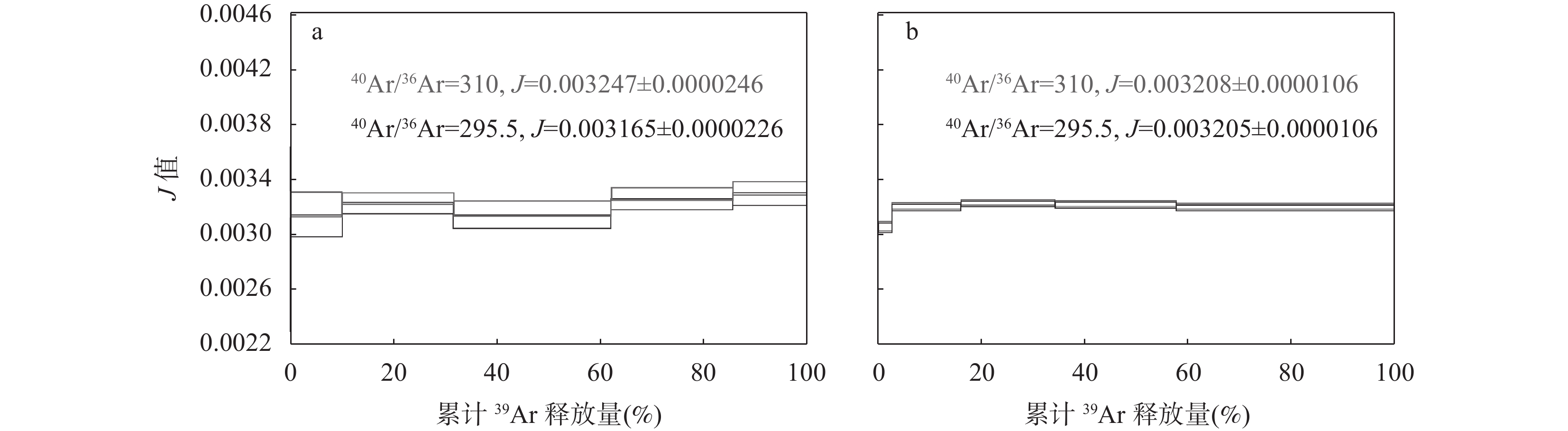
图 1 YBCs透长石A5和A5-1使用大气值(40Ar/36Ar=295.5)和热本底比值(40Ar/36Ar=310)校正大气氩时,对J值计算的影响程度对比。a为在放置14个月的样品盘C内的测试结果,变化幅度约为2.5%; b为置入激光去气后的盘D的测试结果,变化幅度约为0.9‰
Figure 1. Comparison of J-value calculation under different 40Ar/36Ar values with standard YBCs sanidine. a: Results from the oxygen-free copper (OFC) tray exposed to air for fourteen months; J-value changed about 2.5% using 40Ar/36Ar=310 to air correction. b: Results from the reusing OFC tray degassed by laser; J-value changed about 0.9‰.
图 2 系统冷本底、无氧铜盘实际热本底及其同位素比值随激光能量变化的情况。其中,a、b为A盘的结果;c、d为B盘的结果;e、f为D盘的结果。预先经过激光加热的D盘获得了与冷本底一致的40Ar信号量以及40Ar/36Ar比值
Figure 2. The amount of hot blank and isotope ratio characteristic of OFC tray after subtract cold blank. a and b come from tray A; c and d come from tray B; e and f come from tray D. The amount of 40Ar and 40Ar/36Ar value are similar with the cold blank of the system. Green solid circle means cold blank, blue circle means hot blank from first well in copper tray, and red circle means hot blank from second well.
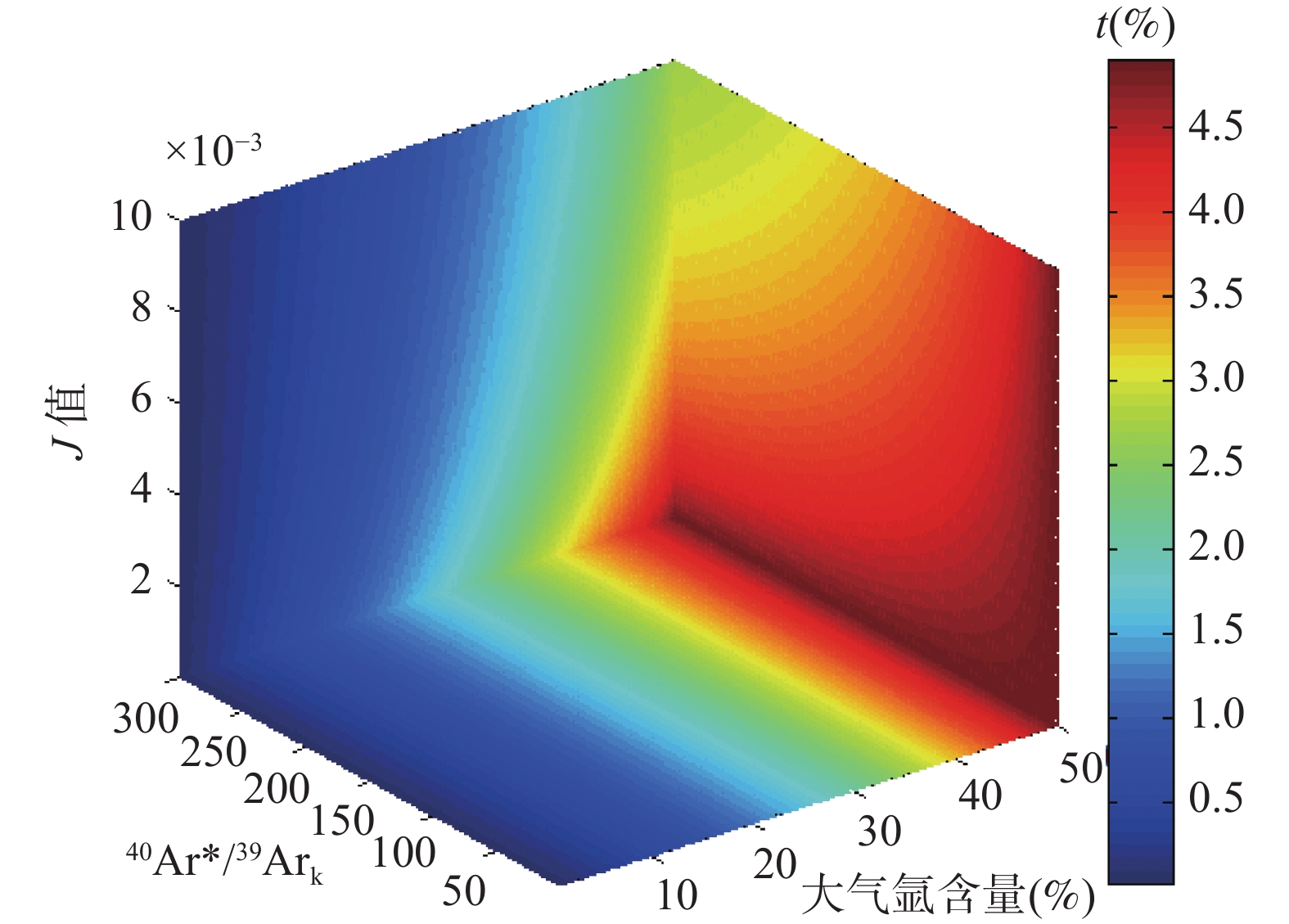
图 3 样品大气氩来源于异常热本底时,使用大气氩初始值(40Ar/36Ar=295.5)扣除样品大气氩,样品年龄偏离真实值的百分比(t/(%))。假定热本底40Ar/36Ar=310;x、y、z轴分别指示模拟计算中样品大气氩含量、40Ar*/39ArK以及 J 值的变化范围
Figure 3. Percent change of 40Ar/39Ar age (t/(%)) resulting from calculating ages with the atmospheric 40Ar/36Ar of 295.5, while sample’s atmospheric argon comes from tray’s abnormal hot blank. Assuming the hot blank has an isotopic composition of 40Ar/36Ar=310; x, y and z axes indicate the range of atmospheric argon content of sample, 40Ar*/39ArK ratio and J-value used in the calculation.
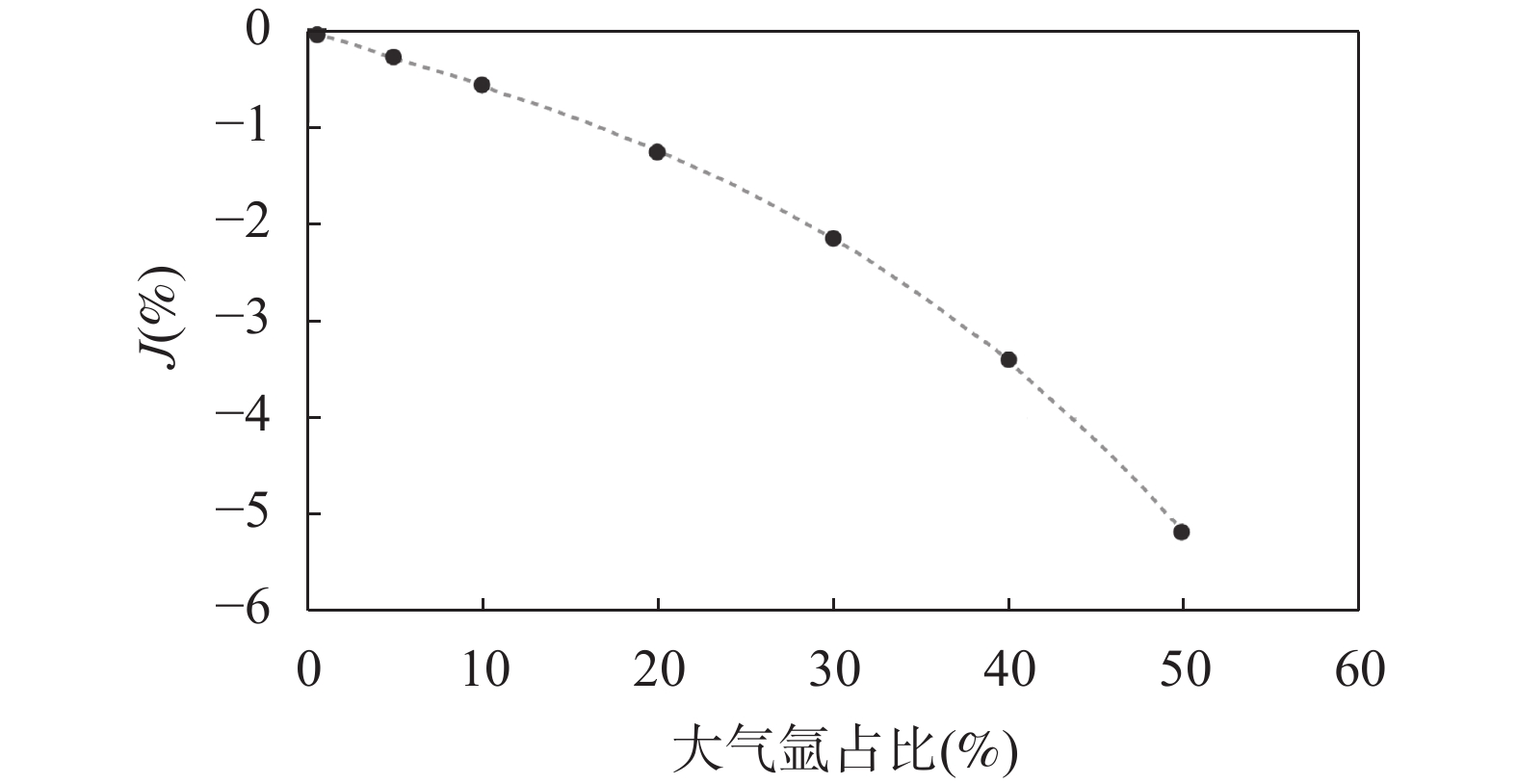
图 4 标准样品大气氩来源于异常热本底时,使用大气氩初始值(40Ar/36Ar=295.5)扣除样品大气氩, J 值偏离真实值的百分比(t/(%))。假定热本底40Ar/36Ar=310, x轴为计算中标准样品大气氩含量的变化范围
Figure 4. Percent change of J-value (J(%)) resulting from calculating J-value with the atmospheric 40Ar/36Ar of 295.5, while standard mineral’s atmospheric argon comes from tray’s abnormal hot blank. Assuming the hot blank has an isotopic composition of 40Ar/36Ar=310, x axe indicates the range of the atmospheric argon content of standard material used in the calculation.
表 1 透长石YBCs样品A5及A5-1测试结果对比:A5显示样品各阶步均含有大量大气氩,A5-1大气氩含量正常(初始氩校正采用40Ar/36Ar=295.5)
Table 1 Result comparison of sanidine YBCs. A5 placed on an OFC tray exposures to air for fourteen months; A5-1 on a reusing tray; data calculation under 40Ar/36Ar=295.5. The proportion of atmospheric argon of steps of A5 is high, and return to normal of A5-1.
透长石YBCs样品A5 激光能量
(W)是否可以形成
坪的阶步40Ar/39Ar 36Ar/39Ar 40Ar*/39Ark 40Ar*(%) 辐照剂量监测 J值 ±2σ 0.24 - 10.319367 0.016838 5.34346 51.78 0.0030628 ±0.0000796 0.39 - 7.088052 0.006638 5.12619 72.32 0.0031926 ±0.0000388 0.54 - 8.306639 0.010221 5.28589 63.63 0.0030961 ±0.0000489 0.70 - 7.192548 0.007138 5.08307 70.67 0.0032197 ±0.0000417 1.32 - 7.205940 0.007393 5.02091 69.68 0.0032595 ±0.0000454 全熔 - - - - 65.59 0.0031648 ±0.0000226 透长石YBCs样品A5-1 激光能量
(W)是否可以形成
坪的阶步40Ar/39Ar 36Ar/39Ar 40Ar*/39Ark 40Ar*(%) 辐照剂量监测 J值 ±2σ 0.16 - 5.363579 0.000023 5.35657 99.87 0.0030553 ±0.0000345 0.39 √ 5.127897 0.000060 5.10986 99.65 0.0032028 ±0.0000205 0.54 √ 5.194142 0.000406 5.07372 97.68 0.0032256 ±0.0000205 0.70 √ 5.312808 0.000752 5.09012 95.81 0.0032152 ±0.0000204 1.32 √ 5.186170 0.000242 5.11425 98.61 0.0032000 ±0.0000194 全熔 - - - - 97.95 0.0032045 ±0.0000106 注:“√”表示可以形成坪的阶步;“-”表示不可以形成坪的阶步。 -
[1] McDougall I, Harrison T M. Geochronology and thermochronology by the 40Ar/39Ar method (The second edition)[M]. New York: Oxford Uninversity Press, 1999: 81-82.
[2] 张佳, 刘汉彬, 李军杰, 等. K-Ar稀释法中40Ar含量测量过程中实验参数的确定[J]. 岩矿测试, 2021, 40(3): 451−459. Zhang J, Liu H B, Li J J, et al. Determination of experimental parameters during measurement of 40Ar content in K-Ar dilution method[J]. Rock and Mineral Analysis, 2021, 40(3): 451−459.
[3] Shi W B, Wang F, Wu L, et al. Geologically meaningful 40Ar/39Ar ages of altered biotite from a polyphase deformed shear zone obtained by in vacuo step-heating method: A case study of the Waziyü detachment fault, Northeast China[J]. Minerals, 2020, 10: 648.
[4] Wang F, He H Y, Zhu R X, et al. Laser step-heating 40Ar/39Ar dating on young volcanic rocks[J]. Chinese Science Bulletin, 2006, 51(23): 2892−2896. doi: 10.1007/s11434-006-2195-9
[5] Barfod D N, Mark D F, Tait A, et al. Argon extraction from geological samples by CO2 scanning laser step-heating[J]. London Geological Society (Special Publications), 2014, 378: 79−90. doi: 10.1144/SP378.23
[6] 高梓涵, 李立武, 王玉慧, 等. 双真空炉管的研制及其在岩石加热脱气气体组分测试中的应用[J]. 岩矿测试, 2019, 38(5): 469−478. Gao Z H, Li L W, Wang Y H, et al. Development of a double vacuum furnace tube and its application in gas composition determination during rock heating degassing[J]. Rock and Mineral Analysis, 2019, 38(5): 469−478.
[7] 张万峰, 邱华宁, 郑德文, 等. 40Ar/39Ar定年自动去气系统的研制及其性能[J]. 地球化学, 2020, 49(5): 509−515. Zhang W F, Qiu H N, Zheng D W, et al. An automatic degassing system for 40Ar/39Ar dating[J]. Geochimica, 2020, 49(5): 509−515.
[8] Wang F, Shi W B, Zhang W B, et al. Multiple phases of mountain building on the Northern Tibetan margin[J]. Lithosphere, 2020: 8829964.
[9] 邱华宁. 新一代Ar-Ar实验室建设与发展趋势: 以中国科学院广州地球化学研究所Ar-Ar实验室为例[J]. 地球化学, 2006, 35(2): 133−140. doi: 10.3321/j.issn:0379-1726.2006.02.003 Qiu H N. Construction and development of new Ar-Ar laboratories in China: Insight from GV-5400 Ar-Ar laboratory in Guangzhou Institute of Geochemistry, Chinese Academy of Sciences[J]. Geochimica, 2006, 35(2): 133−140. doi: 10.3321/j.issn:0379-1726.2006.02.003
[10] McIntosh W C, Heizler M T. Applications of CO2 laser heating in 40Ar/39Ar geochronology[C]//Lanphere M A, Dalrymple G B, Turrin B D. Eighth International Conference on Geochronology, Cosmochronology and Isotope Geology. USA: US Geological Survey Circular 1107, 1994: 212.
[11] Wang F, Jourdan F, Lo C H, et al. YBCs sanidine: A new standard for 40Ar/39Ar dating[J]. Chemical Geology, 2014, 388: 87−97. doi: 10.1016/j.chemgeo.2014.09.003
[12] Koppers A A P. ArArCALC-software for 40Ar/39Ar age calculations[J]. Computers & Geosciences, 2002, 28: 605−619.
[13] Steiger R H, Jäger E. Subcommision on geochronology: Convention on the use of decay constants in geo- and cosmochronology[J]. Earth and Planetary Science Letters, 1977, 36: 359−362. doi: 10.1016/0012-821X(77)90060-7
[14] 杨列坤, 王非, 贺怀宇, 等. 年轻火山岩氩同位素体系定年技术最新进展及问题[J]. 地震地质, 2009, 31(1): 174−185. doi: 10.3969/j.issn.0253-4967.2009.01.016 Yang L K, Wang F, He H Y, et al. Achievements and limitations of 40Ar/39Ar dating on young volcanic rocks[J]. Seismology and Geology, 2009, 31(1): 174−185. doi: 10.3969/j.issn.0253-4967.2009.01.016
[15] 高本辉, 李林. 金属片高温出气[J]. 电子管技术, 1978(2): 136−143. Gao B H, Li L. The sheet metal outgassing characteristics at high temperature[J]. Evacuated Tube Technology, 1978(2): 136−143.
[16] Renne P R, Cassata W S, Morgan L E. The isotopic composition of atmospheric argon and 40Ar/39Ar geochronology: Time for a change?[J]. Quaternary Geochronology, 2009, 4: 288−298. doi: 10.1016/j.quageo.2009.02.015
[17] Nier A O. A redetermination of the relative abundances of the isotopes of carbon, nitrogen, oxygen, argon, and potassium[J]. Physical Review, 1950, 77: 789−793. doi: 10.1103/PhysRev.77.789
[18] Lee J Y, Marti K, Severinghas J P, et al. A redetermination of the isotopic abundances of atmospheric Ar[J]. Geochimica et Cosmochimica Acta, 2006, 70: 4507−4512. doi: 10.1016/j.gca.2006.06.1563
[19] Wang F, Shi W, Guillou H, et al. A new unspiked K−Ar dating approach using laser fusion on microsamples[J]. Rapid Communications in Mass Spectrometry, 2019, 33: 587−599. doi: 10.1002/rcm.8385
[20] Phillips D, Matchan E L, Honda M, et al. Astronomical calibration of 40Ar/39Ar reference minerals using high-precision, multi-collector (ARGUSVI) mass spectrometry[J]. Geochimica et Cosmochimica Acta, 2017, 196: 351−369. doi: 10.1016/j.gca.2016.09.027
[21] Velthaus V, Tietz B, Trautmann C, et al. Desorption measurements of accelerator-related materials exposed to different stimuli[J]. Vacuum, 2021, 194: 110608. doi: 10.1016/j.vacuum.2021.110608
-
期刊类型引用(6)
1. 卢碧翠,张修华,王智青,张红进,刘丽立,潘晓瑜,杨明生,黄中伟,陈奇志. 提高电解二氧化锰中间控制磨粉样铁含量检测效率的研究. 中国锰业. 2024(03): 60-63 .  百度学术
百度学术
2. 杨精存,丁杭冰,施亚菁. 磁性固相萃取-电感耦合等离子体质谱(ICP-MS)法同时检测制革废水多种重金属元素. 皮革与化工. 2024(05): 20-25 .  百度学术
百度学术
3. 冯先进,杨斐. 电感耦合等离子体串联质谱技术特点及国内应用现状. 冶金分析. 2023(09): 1-13 .  百度学术
百度学术
4. 严煜,韩乃旭,卢水淼,夏晓峰,林黎,张秀丽. 工业在线-电感耦合等离子体发射光谱法分析湿法冶炼硫酸锌溶液中铜镉钴铁. 岩矿测试. 2022(01): 153-159 .  本站查看
本站查看
5. 王干珍,彭君,李力,秦毅,曹健,田宗平. 锰矿石成分分析标准物质研制. 岩矿测试. 2022(02): 314-323 .  本站查看
本站查看
6. 王凯凯. 等离子质谱在水环境重金属检测中的应用. 冶金管理. 2021(11): 163-164 .  百度学术
百度学术
其他类型引用(0)



 下载:
下载:



 京公网安备 11010202008159号
京公网安备 11010202008159号