A Review of Research Progress on Separation and Purification Methods of Lithium Isotopes Using Cation Exchange Resin and Their Applications
-
摘要:
锂(Li)同位素是良好地球化学示踪工具,被广泛应用于壳幔物质循环、行星起源与演化、大陆风化、古环境与气候变迁、成矿机制及环境污染等领域。然而,分析测试过程中同质异位素的潜在干扰使得自然样品中Li的高效分离纯化成为关键需求。近几十年来,锂同位素阳离子交换树脂分离纯化法得到了广泛应用和发展,形成了多类型不同自然样品中Li分离纯化的一系列方法体系。从早期Li的分离纯化方法仅注重于实现自然样品Li的分离效果,使之满足TIMS分析需求,逐渐发展到注重减少分离纯化过程中引入空白污染、简化操作提高分离效率以及对不同类型自然样品具有广泛适用性等方面。本文阐述了近年来基于阳离子交换树脂法的锂同位素分离纯化方法研究进展,重点总结了单柱法、双柱法、多柱法和套柱法的操作流程与技术特点,从树脂类型选择及用量、淋洗液类型选择及其在不同类型自然样品中的适用性和应用效果等方面,分析了各类方法的应用优势。目前单柱法和双柱法是Li分离纯化研究中应用较为广泛的方法,在不断优化过程中,一些方法的树脂用量低至1mL,淋洗液用量少于10mL,流程空白控制在1‰以下,整个淋洗流程被缩短至数小时之内。但不同分离纯化方法仍在很大程度上依赖于样品Li含量及其他基质离子等因素。因此,在兼顾分离效率、适用范围以及经济性等因素下,进一步优化现有方法体系、扩展不同类型样品适用范围,对于提升锂同位素分析效率和精度以及推动锂同位素应用研究发展具有重要意义。随着测试设备和技术的不断进步,逐步探索自动化淋洗设备在自然样品Li高效分离纯化中的应用,将成为锂同位素分析技术的主要发展方向之一。
要点(1)锂同位素阳离子交换树脂分离纯化方法在实现样品中Li的100%回收前提下,着重注意样品基质中Na元素的分离。
(2)锂同位素阳离子交换树脂分离纯化方法的优化主要涉及树脂柱高径比、树脂类型及用量、淋洗液类型及用量三个方面。
(3)不同自然储库样品Li含量、锂同位素及其基质组成差异显著,优化分离纯化方法流程并扩展自然样品适用性,对于提升锂同位素分析效率、精度以及推动应用研究非常重要。
HIGHLIGHTS(1) The separation and purification method of lithium isotope via cation exchange resin especially focuses on the separation of sodium in the sample matrix under the premise of 100% recovery of lithium in the sample.
(2) The optimization of separation and purification methods of lithium isotope via cation exchange resin mainly involves three aspects: the ratio of height-diameter of the resin column, the type and dosage of resin and the type and dosage of eluent.
(3) There are significant differences in lithium content, Li isotope and its matrix composition in different natural reservoirs, so it is important to continue to optimize and develop the existing separation and purification process and expand the applicability of natural samples to improve the efficiency and accuracy of Li isotope analysis and promote the application research of Li isotopes.
Abstract:Lithium (Li) isotopes serve as effective geochemical tracers in mantle-crust cycling, planetary evolution, climate change, continental weathering, mineralization mechanisms, and environmental pollution studies. Efficient separation of lithium from natural samples is essential due to potential interference from isobaric isotopes during analysis. Over the past decades, cation exchange resin methods have been developed to enhance lithium separation for TIMS and MC-ICP-MS analysis. Since then, these methods have evolved to reduce blank contamination, simplify procedures, improve efficiency, and expand applicability to various natural samples. This review examines recent advances in Li isotope separation using single-column, double-column, multi-column and in-series column methods. Key factors like resin type, eluent volume, and method efficiency for various samples are discussed. Single- and double-column methods dominate current research, of which some methods just use minimal resin and eluent while controlling process blanks to below 0.1‰. However, separation efficiency remains dependent on lithium content and matrix ions in the sample. Further optimization is needed to balance efficiency, cost, and applicability across sample types. As analytical techniques advance, automated elution systems are likely to become central to Li isotope analysis. The BRIEF REPORT is available for this paper at http://www.ykcs.ac.cn/en/article/doi/10.15898/j.ykcs.202409050181.
BRIEF REPORTLithium (Li) isotopes are effective geochemical tracers and have been widely applied in studies of mantle-crust cycling[1], planetary evolution[2], continent weathering[3], paleoclimate changes[4], mineralization[6], and environmental pollution[7]. As Li isotope research advances, accurate and efficient analysis techniques have become crucial. Since the late 20th century, methods have evolved from NAA[8] and AAS[9] to ICP-MS[10] and TIMS[12-13], achieving precision below 1‰. Recently, in situ techniques like SIMS[17] and LA-MC-ICP-MS[18] have progressed. However, due to the lack of solid standards, MC-ICP-MS[14-16] remains the most widely used technique. Li isotope separation and purification are essential for high-precision analysis, with cation exchange resin methods being mainstream. Various separation methods have been developed based on different resins, eluents, and chromatographic configurations. This review summarizes Li isotope separation techniques over the past thirty years, evaluates their applicability, and provides an outlook on future developments.
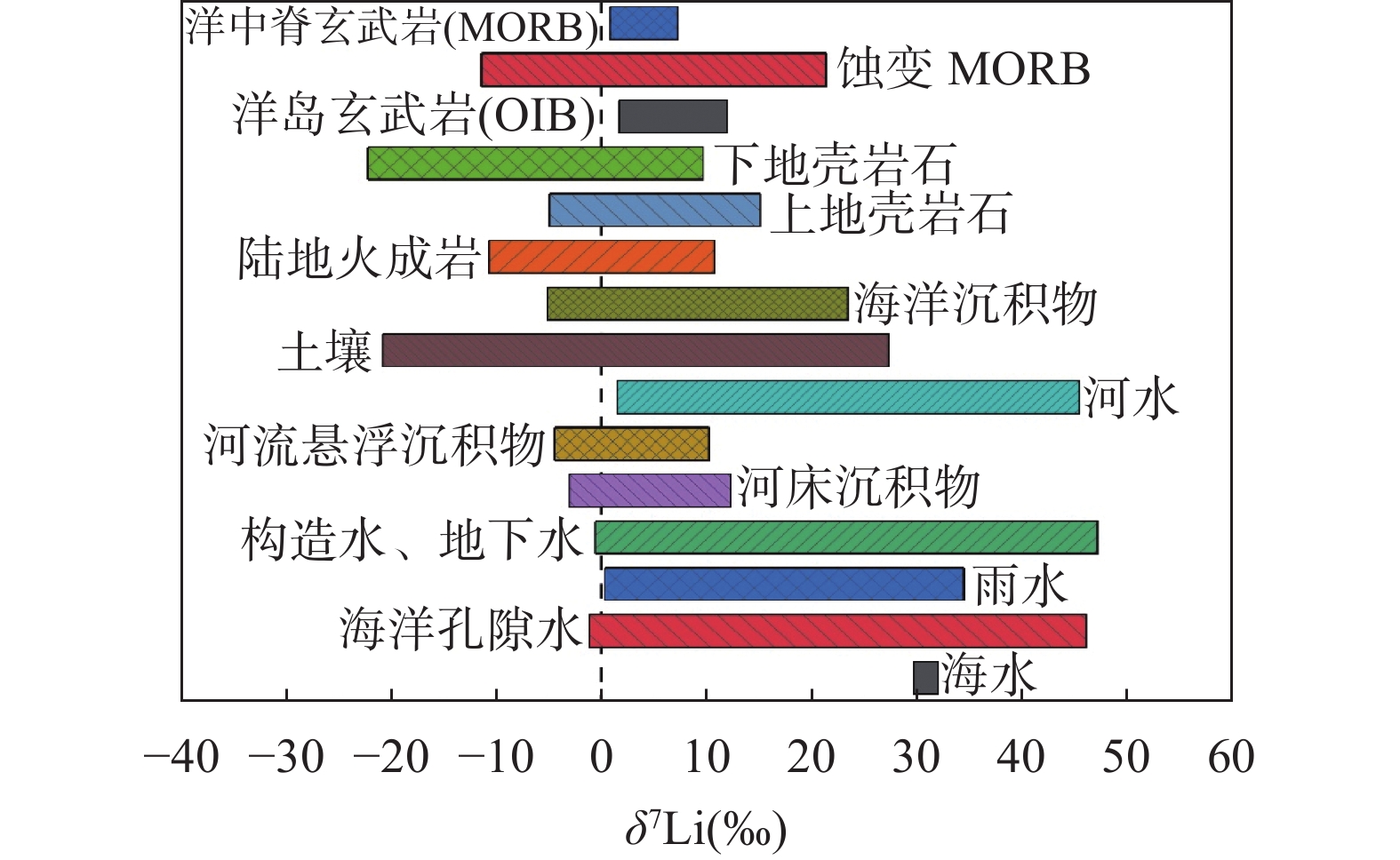
There are distinct Li contents and isotopic compositions in different natural reservoirs. Li exists in two forms in nature: solid, primarily as silicates and Li-bearing minerals, and liquid, widely distributed in natural waters, oceans, and brines. Although Li is a trace element, its content varies significantly across different reservoirs. Its two stable isotopes exhibit significant isotope fractionation during geological processes. Li concentration varies greatly across different reservoirs, ranging from about 1.56μg/L in rivers to 180μg/L in seawater[4], while reaching up to 227000μg/L in brines[25]. In solid reservoirs, Li is concentrated in silicate rocks, with content ranging from 1.49μg/g to 46μg/g[20,26]. Li isotope compositions also vary widely, with seawater at approximately +31‰ and lower in solid reservoirs[4,29]. Due to the similar partitioning behaviors of Li and some other elements, the separation and purification of Li present challenges. Optimized elution processes are required for different sample matrices to ensure efficient Li separation and purification.
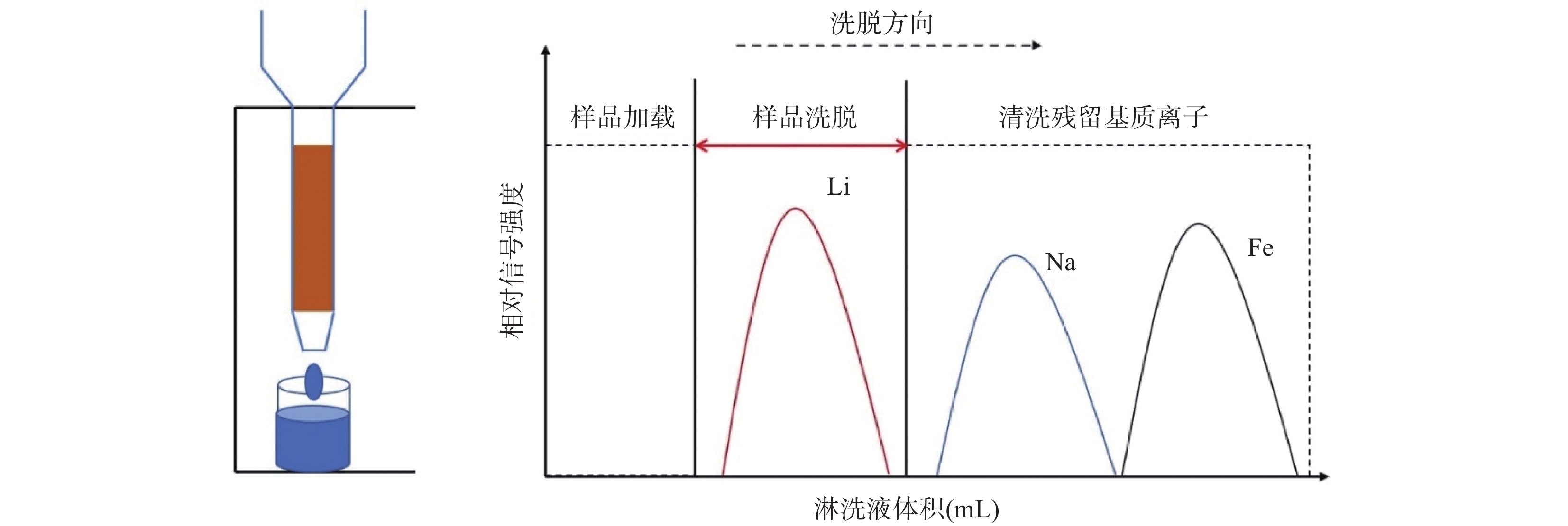
The cation exchange resin method is a common technique for extracting Li from complex matrices, often used in the pretreatment of Li isotope analysis. The process involves dissolving the sample, passing it through a stationary phase (resin) and a mobile phase (acid solution)[37], and using an eluent to separate pure Li. The separation process is based on plate theory, where solute ions maintain dynamic equilibrium between the resin and solution, and are separated by different partition coefficients[37]. Three key factors influence Li isotope separation: the resin's capacity, the type and concentration of eluent, and the amount of resin and eluent used. Additionally, factors such as the type of chromatography column, resin properties, and elution frequency should be considered to avoid contamination and optimize the purification process[32,39-40].
1. Different separation and purification methods of Li via cation exchange resin and their technical characteristics
In the separation and purification methods of Li via cation exchange resin, researchers select appropriate resins, eluents, and chromatography columns based on the composition of the samples. Focusing on elution column numbers as the key variable, this comprehensive review discusses four commonly used methods, single-column, double-column, multi-column, and in-series column, and also analyzes their technical features and applications.
1.1 Single-column method
The single-column method achieves complete elution of Li using a single column and a one-time elution process. Typically, higher columns are used to increase the plate number and improve separation efficiency. In recent years, this method has become the primary approach for Li isotope separation and purification due to its simplified operation and fewer sample transfer steps[52-54]. However, over time, significant differences in the choice of columns and eluents have emerged. Early methods often employed acid-alcohol mixed eluents[14-15], which enhanced the separation between Li and Na but introduced challenges such as large eluent volumes, longer processing times, and higher procedural blanks. Additionally, alcohol-based solutions could degrade the resin, releasing impurities like sodium and further affecting purification[55]. Recently, Zhu et al[45] improved this method by using AGMP-50 resin and replacing alcohol-based eluents with simpler hydrochloric or nitric acid solutions, greatly reducing elution time and eluent volume while minimizing the procedural blanks. This refined method is not only applicable to various natural samples, including seawater and rocks, but also ensures a high Li recovery rate of up to 99%, ensuring both efficiency and accuracy in separation and purification.
1.2 Double-column method
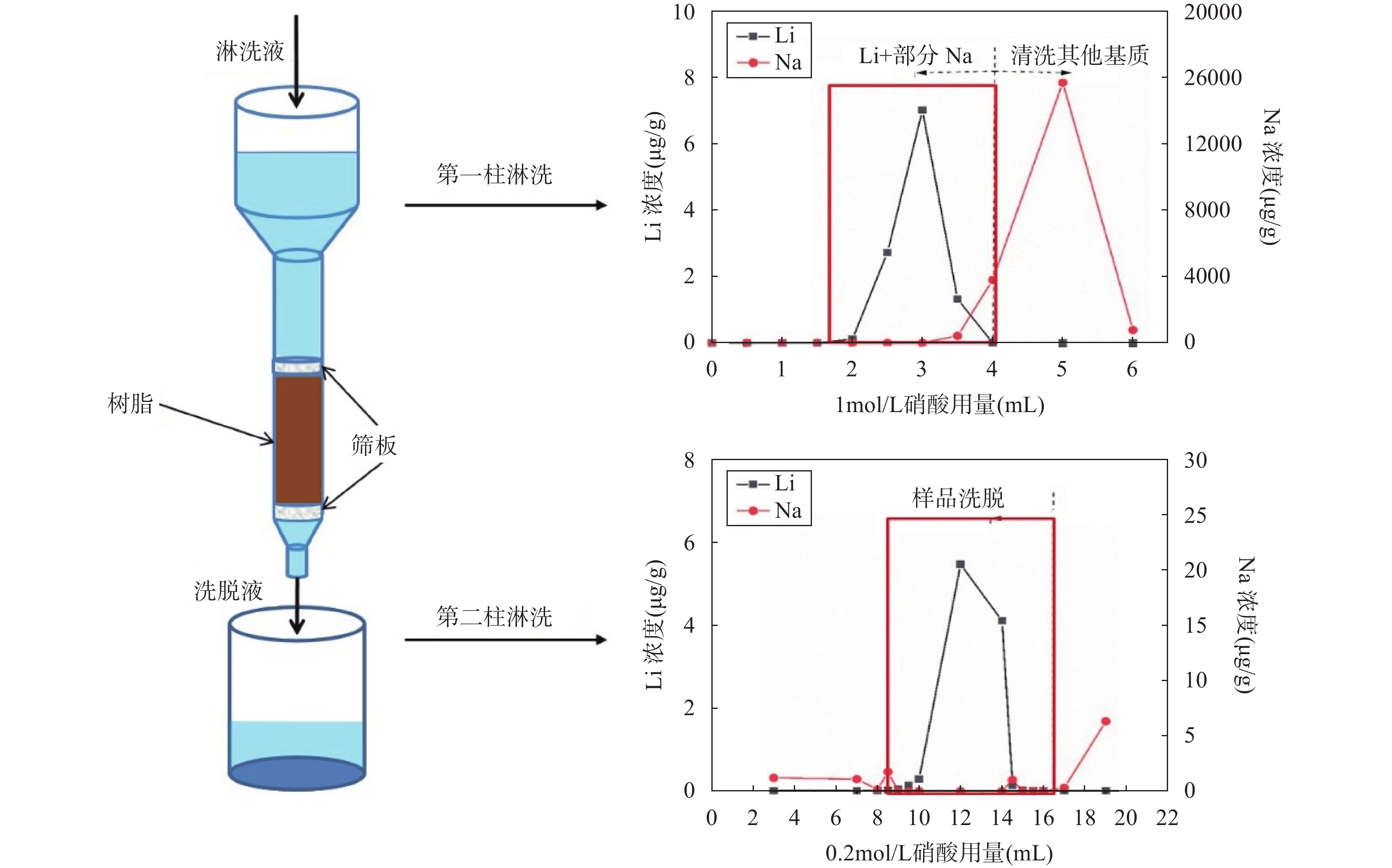
The dual-column method was originally designed to address the challenge of separating Li from Na due to their similar partition coefficients without using alcohol-based eluents. The process is divided into two steps: the first separates most cations from Li; the second step ensures the complete separation of Li from Na. Since this method uses columns with a small height-to-diameter ratio, the elution time is relatively short, making it a widely used approach alongside the single-column method.
James et al[46] firstly developed the application of the dual-column method, using 2.7mL of AG50W-X12 resin and 0.2mol/L HCl as the eluent. Through two repeated elution, they achieved complete purification of Li, providing a foundation for subsequent improvements. Zhang et al[31] optimized this method by switching to HNO3 instead of HCl to avoid the formation of complexes with trivalent iron, which could interfere with Li separation. They also reduced the amount of resin, making the process more efficient, with significant reductions in both elution time and eluent volume. In recent years, Li et al[48], Zhang et al[44], and Zhu et al[45] have further refined the dual-column method, developing procedures suitable for different types of natural samples.
1.3 Multi-column method
The multi-column method was initially developed to meet the high-purity requirements of Li during isotope analysis in TIMS while minimizing the use of resin and eluent to avoid Li blank contamination. This method employs multiple columns or repeated elution steps, using small amounts of resin to gradually remove matrix ions, ultimately achieving 100% purification of Li. In the late 20th century, Moriguti et al[22] developed a four-step separation method using different concentrations of hydrochloric acid and hydrochloric acid-ethanol mixtures for multiple elution to successfully separate Li. Later, Rudnick et al[58] simplified this process by retaining only the first three steps and reducing the acid concentration to improve efficiency. Su et al[39] and Zhao et al[50] further optimized the method by reducing the eluent volume and increasing separation efficiency. Consequently, research on improving this method has been limited in recent decades, and its application is less widespread compared to the single-column and dual-column methods.
1.4 In-series column method
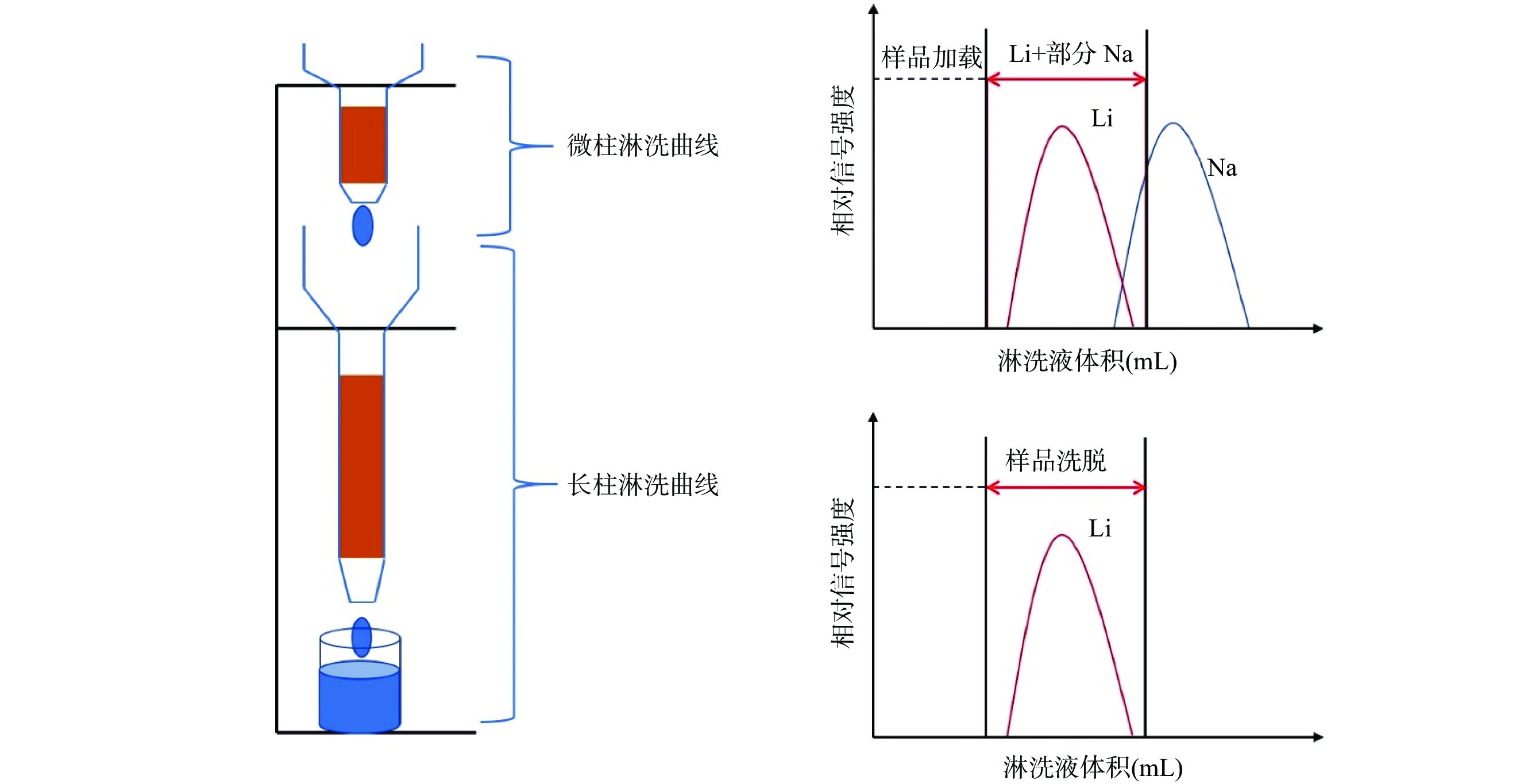
The in-series column method achieves Li separation and purification in a single elution by connecting micro-column and long column in series. The micro-column efficiently adsorbs cations, while the long column further separates Li from Na, ensuring high recovery (>96%). Compared to the single-column and dual-column methods, the in-series method has been applied later, and its technique still requires optimization. Zhu et al[51] found that traditional single-column methods are prone to interference from matrix elements, affecting Li purification efficiency and isotope accuracy. To address this, Zhu et al[51] optimized elution conditions using 0.5mol/L HCl and increased resin capacity, significantly reducing interference and shortening the elution time to around 2h. This method improves elution efficiency, avoids the negative effects of organic solvents, and reduces costs. After validation, the method demonstrated excellent accuracy across various sample types, especially for low-Li and high-matrix samples.
2. The applicability of different separation and purification methods for natural samples
The widespread application of Li isotopes in environmental, geological, and geochemical studies relies on accurate and efficient analysis[59], which requires sample separation and purification based on varying Li content and matrix composition. The Li content and matrix composition differ significantly across reservoirs. For example, Li content is higher in silicate rocks, while carbonate rocks contain higher levels of Ca, Mg and lower content of Li. Among existing Li separation methods, single-column and dual-column methods are the most widely used. Early single-column methods based on nitric acid-methanol[14-15,41] have been replaced by HCl elution[42,56], which is more suitable for high-Li samples. Dual-column methods show advantages in handling samples with high Mg/Fe ratios but low Li content[47]. The in-series column method, as an emerging approach, is applicable to a variety of natural samples and holds promising development prospects.
3. Concluding remarks
The cation exchange resin method has been sufficiently developed to make significant progress in the separation and purification of Li isotopes. The eluent has evolved from mixed inorganic acids and alcohols to purely inorganic acids, reducing the volume from nearly 300mL to less than 10mL. Today, the purification process for natural samples takes only a few hours, with a precision better than 1‰ and process blanks below 1‰. The single-column method is simple and widely used for high-Li, low-matrix samples; the dual-column method excels with complex matrices, especially high-Na samples; and the in-series column method is highly effective for low-Li, high-matrix samples, being both environmentally friendly and economical. Multi-column methods have been phased out due to complexity.
4. Future perspectives
Future research should focus on two areas: first, optimizing and innovating separation and purification methods to improve resin selectivity, streamline operations, reduce procedural blanks, and enhance Li recovery and analytical accuracy, particularly for extreme environmental samples and trace Li analysis. Second, as testing technologies advance, automated elution systems are expected to enable more efficient Li separation and purification, becoming a major direction for isotope analysis.
-
硅元素被国际土壤界认为继氮、磷、钾之后的第四种植物营养元素[1]。硅对植物的形态特征、生理特征和植物体内其他营养元素的分布有一定影响。硅可以促进植物生长,提高光合作用,提高根系活力,增强抗病能力,提高植物产量等[2]。土壤中硅的含量差异较大,形态也多种多样,主要有石英、二氧化硅以及硅酸盐或铝硅酸盐,其含量测定尤为重要[3-4]。
土壤和沉积物中硅含量测定的报道诸多,主要有分光光度法、X射线荧光光谱法(XRF)、激光诱导击穿光谱技术和电感耦合等离子体发射光谱法(ICP-OES)[5-10]。分光光度法操作繁琐、要求高,不适合批量样品测试[11]。行业标准《土壤和沉积物 无机元素的测定 波长色散X射线荧光光谱法》(HJ 780—2015)中采用XRF法需要高温熔融制样[12],并且建立校准曲线比较繁琐。激光诱导击穿光谱技术的测试结果与标准值基本吻合[13],准确度有待提高。ICP-OES法具有分析速度快、线性范围宽、检出限低、准确度高,并且能同时分析多种元素的优点,得到了广泛应用。ICP-OES法测定硅含量的前处理方法主要有碱性熔剂熔融制样或多种混合酸消解样品。行业标准《土壤和沉积物 11种元素的测定 碱熔-电感耦合等离子体发射光谱法》(HJ 974—2018)采用碱性熔剂高温熔融,酸解后利用ICP-OES法测定土壤和沉积物中硅含量。王龙山等[14]报道了一种采用高温熔融,超声提取ICP-OES测定岩石、水系沉积物和土壤样品中硅含量的方法。余浪等[3]报道了一种采用盐酸-硝酸-氢氟酸混合酸,100~110℃微波消解样品,以铑为内标,基体匹配法测定硅含量的方法。杨娜等[15]采用微波消解,ICP-OES法测定硅含量。采用ICP-OES法测定硅含量时,碱熔法引入大量盐,测试时会有基体干扰;浸提法只能测定有效硅含量;微波消解设备相对昂贵;传统消解方法易造成硅挥发损失。因此,亟需开发一种前处理简单、效率高、准确度高、经济实惠的测定土壤中全硅含量的方法。
用超声作为样品前处理有诸多报道,包括超声消解电感耦合等离子体质谱法(ICP-MS)测定烟丝中钾钠钙镁元素含量[16]、超声辅助逆王水提取ICP-MS测定PM2.5颗粒物中24种金属元素含量[17],以及超声波水浴消解ICP-MS法测定土壤中Mn、Co、As、Ag、Cd、Sb和Bi元素含量[18]。在这些报道中,主要采用超声提取或超声辅助半消解测定样品中部分元素,这些元素稳定并且比较容易提取。本文在超声消解基础上,考虑混合酸消解时氢氟酸与硅反应生成的氟化硅易挥发损失、难以准确测定的特点,采用稀王水-氢氟酸-双氧水在密闭条件下超声消解样品,并对是否超声、是否密闭、超声条件及双氧水的加入量进行探讨,采用ICP-OES测定硅元素含量。将建立的方法测定国家标准物质GBW07401a(GSS-1a)、GBW07405a (GSS-5a)、GBW07377 (GSD-26)、GBW07379 (GSD-28)中硅含量,并与XRF结果进行对比,验证了方法的准确性和可靠性。
1. 实验部分
1.1 仪器与工作参数
Avio 500型电感耦合等离子体发射光谱仪(美国PerkinElmer公司);超纯水机(MILLI-Q ADVANTRGE A10);超声波清洗机(春霖公司)。
仪器谱线范围163~782nm;耐氢氟酸系统;功率1400W;进样量1.5mL/min;等离子气流速12L/min;辅助气流速0.5L/min;雾化气流速0.7L/min;径向观测方式。硅的分析谱线有251.611nm、212.412nm、288.158nm、252.851nm等,根据分析谱线的选取原则,分析应该选择灵敏度高、干扰少、线性范围宽的谱线,同时参照仪器推荐,最终选取元素的分析谱线为Si 251.611nm。
1.2 主要试剂
硝酸(CMOS纯,高纯半导体级);盐酸(CMOS纯,高纯半导体级);氢氟酸(CMOS纯,高纯半导体级);双氧水(优级纯);硅标准溶液(1000mg/L):购自国家有色金属及电子材料分析测试中心。
1.3 样品和标准物质
土壤实际样品:在云南松林内设置一块20m×20m样地。在样地内按照S形路线,选取9个点,采集0~10cm表层土壤样品混合成一份样品,每份样品进行编号。样品带回实验室进行风干,去除石块和根系,研磨,过100目筛,部分样品用于测试分析。编号分别为8、18、23、28、31作为实际样品测试。
标准物质GBW07401a (GSS-1a):暗棕壤,黑龙江西林铅锌矿区土壤,采用XRF法及重量法定值。GBW07405a (GSS-5a):黄红壤,江西七宝山多金属矿区土壤,采用XRF法及重量法定值。GBW07377 (GSD-26):水系沉积物成分标准物质,西藏纳木错沉积岩区,采用容量法定值。GBW07379 (GSD-28):水系沉积物成分标准物质,云南兰坪铅锌矿区,采用容量法定值。以上标准物质都是中国地质科学院地球物理地球化学勘查研究所研制。
1.4 样品溶液和标准溶液的制备
样品溶液:准确称取 0.05~0.10g样品置于 50mL离心管中,精确至0.0001g,加入20mL水润湿,加6mL王水、6mL氢氟酸、6mL双氧水,密封后于75℃超声1h,待溶液冷却至室温后转移至1000mL塑料容量瓶中,用超纯水定容后摇匀,待用。若浓度过高,用超纯水稀释后测定样品;若有不溶物,静置过夜后取上清液测定样品。
标准溶液:分别移取硅元素标准溶液(1000mg/L)0、0.5、1、1.5、2、3、5mL于6支100mL容量瓶中,分别加入2%硝酸定容,配制成浓度为0、5、10、15、20、30、50mg/L系列的标准溶液。
2. 结果与讨论
2.1 样品前处理对硅含量测试结果的影响
土壤和沉积物中硅含量测定方法是:样品先与碱性熔剂熔融,熔融物经酸溶解后用ICP-OES进行测定。样品前处理操作繁琐,且熔融过程引入了大量碱金属,测定时基体效应明显。本文采用超声密闭混合酸(稀王水-氢氟酸-双氧水)消解土壤和沉积物中的硅,测定时用耐氢氟酸系统,并且定容至1000mL减少基体效应。为确定密闭条件、超声条件及双氧水的加入量对样品前处理的影响,进行了不同消解条件下的对比实验。
2.1.1 不同消解条件对样品测试结果的影响
以GBW07401a (GSS-1a)为例,在相同的酸浓度、密封条件及反应时间(1h)内,对样品分别进行静置、75℃加热及75℃超声处理,测试结果列于表1,并计算测试结果的相对误差。样品溶液加酸后,静置条件下测试结果明显偏低;75℃加热时测试结果有所提高,但测试结果仍偏低;75℃超声处理样品时,测定值与理论值符合。超声的空化作用及非线性效应有利于样品溶液的分散及促进化学反应进行,加速样品消解。因此,消解土壤和沉积物样品中的硅时,需要在75℃下超声处理样品。
表 1 不同消解条件下样品测试结果的比较Table 1. Comparison of results with different digestion conditions序号 实验条件 SiO2含量测定值
(%)RSD
(%)以SiO2计平均值
(%)SiO2含量认定值及
不确定度(%)以SiO2计相对误差
(%)1 静置 39.86 39.90 40.26 0.6 40.01 56.60±0.46 −29.31 2 75℃加热 51.13 51.34 50.92 0.4 51.13 56.60±0.46 −9.67 3 75℃超声 57.29 56.41 55.71 1.4 56.47 56.60±0.46 −0.23 2.1.2 密闭效果对样品前处理的影响
为确定密闭效果对样品前处理的影响,以GBW07401a(GSS-1a)为例,在相同的酸浓度、超声温度及时间内(1h),进行了敞口、半封闭、密闭不同条件下处理样品,测试结果与理论值的相对误差越来越小(表2)。敞口或半封闭条件下消解样品,测试结果偏低;全封闭效果较好。可能是因为敞口或半封闭时,反应生成的四氟化硅易挥发损失,导致测试结果偏低,而密封条件下避免了硅的损失。超声消解样品,密闭条件下压力增加,与非密闭条件相比,相当于增加了温度和压力,样品在温度与压力的双重作用下消解速率加快,反应时间减少。因此,消解土壤和沉积物样品时需要采用密闭条件。
表 2 密闭条件对样品消解效果的影响Table 2. Influence of different sealing conditions on sample digestion序号 密闭方式 SiO2含量测定值
(%)RSD
(%)以SiO2计平均值
(%)SiO2含量认定值和
不确定度(%)以SiO2计相对误差
(%)1 敞口 49.14 49.14 48.84 0.3 49.04 56.60±0.46 −13.36 2 半密封 51.07 50.92 50.02 1.1 50.67 56.60±0.46 −10.48 3 密闭 57.29 56.41 55.71 1.4 56.47 56.60±0.46 −0.23 2.1.3 超声温度对样品前处理的影响
为确定超声温度对样品前处理的影响,以GBW07401a(GSS-1a)为例,在相同的酸浓度、密封条件及反应时间(1h)内,对样品分别进行不同温度下超声处理,测试结果列于表3,并计算测试结果的相对误差。室温(25℃)及45℃超声处理后,测试结果均偏低;75℃超声1h,测试结果与理论值相符;温度升高至85℃,测试结果与理论值基本相符。低温条件下超声对消解效果影响不明显,可能只是分散作用;随着超声温度升高,超声与加热的双重作用使消解速率逐渐加快。温度太低不利于消解反应的进行,需要很长时间才能消解完全;温度太高,可能会影响离心管密封效果,导致结果偏低。因此,消解土壤和沉积物样品中的硅时选择75℃。
表 3 超声温度对样品消解效果的比较Table 3. Comparison of results with different ultrasound temperature序号 超声温度
(℃)SiO2含量测定值
(%)RSD
(%)Si含量平均值
(%)以SiO2计平均值
(%)SiO2含量认定值和
不确定度(%)以SiO2计相对误差
(%)1 25 40.48 40.82 40.82 0.5 19.03 40.71 56.60±0.46 −28.07 2 45 45.59 45.46 44.88 0.8 21.18 45.31 56.60±0.46 −19.95 3 75 57.29 56.41 55.71 1.4 26.40 56.47 56.60±0.46 −0.23 4 85 55.45 56.71 56.18 1.1 26.23 56.11 56.60±0.46 −0.87 2.1.4 超声时间对样品前处理的影响
为确定超声时间对样品前处理的影响,以GBW07401a(GSS-1a)为例,在相同的酸浓度、密封条件及反应温度下,对样品分别进行不同超声时间处理,测试结果列于表4,并计算相对误差。75℃下超声0.5h,样品大部分已消解完全,超声1h样品已消解完全。随着超声时间延长,消解结果逐渐完全并保持稳定,测试结果与理论值越来越接近,相对误差也越来越小。为节省时间,选择样品溶液超声1h。
表 4 超声时间对样品前处理效果的比较Table 4. Comparison of results with different ultrasound time序号 超声时间
(h)SiO2含量测定值
(%)RSD
(%)Si含量平均值
(%)以SiO2计平均值
(%)SiO2含量认定值和
不确定度(%)以SiO2计相对误差
(%)1 0.5 50.34 49.68 50.38 0.8 23.43 50.13 56.60±0.46 −11.43 2 1 57.29 56.41 55.71 1.4 26.40 56.47 56.60±0.46 −0.23 3 2 57.08 57.10 56.22 0.9 26.55 56.80 56.60±0.46 0.35 4 3 56.26 56.50 56.29 0.2 26.34 56.35 56.60±0.46 −0.44 2.1.5 超声功率对样品前处理的影响
为确定超声功率对样品前处理的影响,以GBW07401a(GSS-1a)为例,在相同的酸浓度、密封条件、超声温度及超声时间内,对样品进行不同功率超声处理,测试结果列于表5,并计算测试结果的相对误差。随着超声功率增加,硅含量测试值逐渐增大,并与理论值越来越接近,测试值的相对误差也越来越小。当超声功率增大到一定值,测试结果与理论值相符。因此,消解样品时选择300W功率。
表 5 超声功率对样品前处理效果的测试结果比较Table 5. Comparison of results with different ultrasound powder序号 超声条件 Si含量测定值
(%)RSD
(%)Si含量测定平均值
(%)以SiO2计平均值
(%)SiO2含量认定值
及不确定度(%)以SiO2计相对误差
(%)1 75℃超声功率120W 56.44 55.64 55.39 1.0 26.09 55.82 56.60±0.46 −1.38 2 75℃超声功率240W 55.90 55.97 55.75 0.3 26.10 55.87 56.60±0.46 −1.29 3 75℃超声功率300W 57.29 56.41 55.71 1.4 26.40 56.47 56.60±0.46 −0.23 4 75℃超声功率360W 56.50 56.95 56.97 0.5 26.55 56.80 56.60±0.46 0.35 2.1.6 双氧水对样品前处理效果的影响
超声条件确定后,为确定双氧水对样品消解的影响,以GBW07401a(GSS-1a)为例,在其他条件相同的情况下,进行了不同添加量的双氧水对样品消解对比实验,测试结果见表6,并计算测试结果的相对误差。相同超声条件下,不加双氧水时,测试结果明显偏低;加入3mL双氧水后,测试结果有一定程度提高,但还是低于理论值;加入6mL双氧水和加入9mL双氧水后测试结果均与理论值相符,因此选择加入6mL双氧水。
表 6 不同添加量的双氧水对样品进行消解测试结果比较Table 6. Comparison of results with different amounts of hydrogen peroxide added序号 双氧水用量
(mL)Si含量测定值
(%)RSD
(%)Si含量测定平均值
(%)以SiO2计平均值
(%)SiO2含量认定值
及不确定度(%)以SiO2计相对误差
(%)1 0 42.89 42.25 42.44 0.8 19.88 42.53 56.60±0.46 −24.86 2 3 50.30 48.97 48.50 1.9 23.02 49.26 56.60±0.46 −12.97 3 6 57.29 56.41 55.71 1.4 26.40 56.47 56.60±0.46 −0.23 4 9 55.92 55.73 56.78 1.0 26.24 56.14 56.60±0.46 −0.81 采用稀王水-氢氟酸-双氧水消解试样,75℃密闭条件下超声,可发生的反应有:
HNO3+3HCl= 2H2O+Cl2+NOCl
H2O2+HNO3=HNO2+H2O+O2↑
2H2O2+2HCl=2HClO+2H2O=O2+2HCl+2H2O
Cl2+H2O2=2HCl+O2
3mL盐酸和1mL硝酸加热时,生成的氯化亚硝酰和新生的氯气具有较强的氧化性;双氧水与硝酸反应生成的亚硝酸,它的氧化能力在稀溶液时比NO3−离子还强;双氧水与盐酸反应生成的次氯酸具有很强的氧化性,可以把盐酸氧化成氯气;因此,反应生成的各种氧化性物质与酸的作用加速了样品溶解,并且土壤和沉积岩样品分解后的大多数矿物生成氯化物或氯配离子转入溶液,氯离子的配位作用进一步加速了样品溶解。稀酸处理样品,生成的氟化硅与水反应生成氟硅酸和硅酸。3SiF4+4H2O=2H2SiF6+H4SiO4,促进大量硅元素进入水溶液,减少了SiF4气体含量,抑制硅元素损失。采用稀王水-氢氟酸-双氧水消解试样,大大提高了反应效率,缩短反应时间,简化了前处理操作。表明土壤和沉积物中硅的测定可采用稀王水-氢氟酸-双氧水在全封闭条件下,75℃超声1h,测试结果准确。
2.2 校准曲线和方法检出限
将配制好的标准溶液在仪器工作条件下进行测定,分析线251.611nm,以Si元素浓度为横坐标、强度为纵坐标,采用线性计算截距的方式,绘制标准曲线。Si元素线性回归方程为:y=3573.41847x−430.08986,相关系数为0.999974,在5~50mg/L范围内线性良好。在最优工作条件下,按照实验方法,连续测定11次2%硝酸空白溶液,硅含量测试结果(mg/L)分别为:0.145、0.147、0.146、0.145、0.146、0.145、0.146、0.145、0.144、0.144、0.144。以测试结果的3倍标准偏差乘以稀释倍数(按称重0.1g,定容到1000mL),计算方法检出限为0.0395mg/g。
2.3 方法精密度和准确度验证
选取不同种类的国家标准物质GBW07401a、GBW07405a、GBW07377、GBW07379进行测试,每个标准物质平行分析 11 次。计算平均值与标准值之间的相对误差(%)来衡量方法准确度;计算 11 次平行测定的相对标准偏差(RSD)来衡量方法精密度。由表7可知,RSD在 0.26%~0.54%,说明方法精密度良好。ICP-OES测定值与标准值的相对误差在−0.28%~0.25%,说明方法准确度良好。
表 7 方法精密度和准确度实验Table 7. Precision and accuracy tests of the method标准物质编号 Si含量测定值
(%)RSD
(%)Si含量测定
平均值(%)以SiO2计
平均值(%)SiO2含量认定值
及不确定度(%)以SiO2计
相对误差(%)GBW07401a 56.91 56.80 56.84 0.40 26.52 56.74 56.60±0.46 0.25 56.22 56.93 56.65 56.99 56.71 56.91 56.69 56.50 GBW07405a 61.48 61.18 61.81 0.31 28.68 61.35 61.52±0.39 −0.28 61.16 61.33 61.25 61.21 61.21 61.44 61.36 61.38 GBW07377 63.50 63.17 63.75 0.26 29.65 63.43 63.48±0.43 −0.08 63.52 63.47 63.43 63.35 63.28 63.56 63.24 63.50 GBW07379 69.38 69.98 69.21 0.54 32.62 69.75 69.66±0.6 0.13 70.15 69.79 70.08 69.87 70.17 69.76 69.12 69.70 2.4 不同分析方法测试结果比对
为确定该方法的实用性,选取了不同硅含量的土壤实际样品,样品编号分别为8、18、23、28、31,每个样品称取5个平行样品,用本文的超声法快速消解样品并测试,同时用XRF法进行测定(三次测定,给出平均值)。由表8测试结果可知,实际样品两种方法比对发现,结果有部分偏差,两种方法的相对误差在−12.6%~27.1%,说明这两种方法测定结果偏差较大。而采用本文方法测定不同硅含量土壤样品的RSD为0.52%~0.77%,测试结果精密度良好,表明本文方法适用于实际样品测试。
表 8 实际样品测试结果比对Table 8. Comparison of analytical results of SiO2 content in actual samples实际样品编号 本文方法Si含量测定值
(%)RSD
(%)Si含量测定平均值
(%)XRF法Si含量测定值
(%)相对误差
(%)样品8 29.98 29.72 30.18 29.68 29.90 0.68 29.89 26.12 −12.60 样品18 12.13 12.03 12.22 12.04 12.06 0.66 12.10 15.38 27.10 样品23 14.91 15.11 14.95 14.93 15.17 0.77 15.01 17.07 13.70 样品28 29.93 29.75 29.80 29.86 30.15 0.52 29.90 27.67 −7.46 样品31 31.81 31.65 31.96 31.66 31.54 0.52 31.72 29.28 −7.69 对国家标准物质GBW07401a、GBW07405a、GBW07377、GBW07379也同时采用XRF法测定(三次测定,给出平均值)进行测试比对。由表9测试结果可知,本文方法与XRF测试结果有部分偏差,相对误差在−0.65%~4.80%。根据行业标准《土壤和沉积物 无机元素的测定 波长色散X射线荧光光谱法》(HJ 780—2015)可知,国家有证标准物质中元素含量在5%以上时,误差要求在5%以内,除了GBW07405a在认定值范围内,其余三个标准物质均在XRF测量误差范围以内。
表 9 标准物质测试结果比对Table 9. Comparison of analytical results of SiO2 content in national standard substances标准物质编号 本文方法Si含量
测定平均值(%)RSD
(%)XRF法Si含量
测定值(%)以SiO2计XRF法
测定值(%)SiO2含量认定值
及不确定度(%)以SiO2计本文方法与
XRF法相对误差(%)GBW07401a 26.52 0.40 27.69 59.24 56.60±0.46 4.66 GBW07405a 28.68 0.31 28.57 61.12 61.52±0.39 −0.65 GBW07377 29.65 0.26 31.10 66.53 63.48±0.43 4.80 GBW07379 32.62 0.54 33.17 70.96 69.66±0.6 1.87 3. 结论
建立了超声快速消解ICP-OES法快速测定土壤和沉积物中硅元素含量的分析方法。通过优化样品前处理条件,对密闭条件、超声时间、超声温度及双氧水加入量进行筛选,选择合适的分析谱线,测定了土壤和沉积物国家标准物质GBW07401a、GBW07405a、GBW07377、GBW07379中的硅含量,并进行了精密度及准确度实验,其相对标准偏差(RSD)在 0.26%~0.54%,相对误差在−0.28%~0.25%。并通过实际样品测试,验证了本文方法的适用范围。
与XRF法测试结果对比,本文方法操作简便、成本低,适用于大批量样品中易挥发元素硅的测定,对于其他易挥发元素的测定需要进一步探索。
-
图 2 色谱柱法锂的洗脱流程示意图(修改自Schonbachler等[37])
Figure 2. Schematic diagram of lithium elution process by column chromatography (Modified from Schonbachler, et al[37]): (a) Lithium is completely adsorbed by the resin; (b) Lithium migrates downward in the resin column with the eluent; (c) Lithium is completely eluted and separated.
表 1 不同类型锂分离纯化方法中色谱柱、树脂、淋洗介质及淋洗方式
Table 1 Chromatographic column, resin, eluent and elution type in the different lithium separation and purification methods
序号 色谱柱(尺寸) 树脂(用量) 淋洗介质 淋洗方式 参考文献 1 派热克斯玻璃柱(柱高>20cm) AG 50W-X8 0.5mol/L盐酸 单柱 [12] 2 石英柱 (柱高30cm) AG 50W-X8 1mol/L硝酸的80%甲醇混合溶液 单柱 [14] 3 石英柱 (柱高12.5cm) AG 50W-X8 0.5mol/L硝酸的80%甲醇混合溶液 单柱 [41] 4 聚四氟乙烯柱(柱高>10cm) AG 50W-X8 1mol/L硝酸的75%甲醇混合溶液 单柱 [15] 5 石英柱 (柱高30cm) AG 50W-X8 0.15mol/L盐酸的30%乙醇混合溶液 单柱 [32] 6 聚四氟乙烯柱
(柱高5cm)AG 50W-X8
(2mL)0.5mol/L盐酸 单柱 [42] 7 硼硅玻璃柱
(柱高21.5cm)AG 50W-X8
(2mL)顺序加入:0.67mol/L硝酸的30%甲醇混合溶液、
1 mol/L硝酸的80%甲醇混合溶液单柱 [43] 8 Savillex-聚四氟乙烯
(柱高20cm)AG 50W-X12
(1.5mL)顺序加入:0.4mol/L盐酸、1.0mol/L盐酸 单柱/双柱 [44] 9 聚丙烯柱
(柱高8.5cm)AGMP-50
(约1.4g)顺序加入:0.2mol/L盐酸、0.73mol/L盐酸和
0.3mol/L 氢氟酸单柱/双柱 [45] 10 聚四氟乙烯柱(柱高>8.5cm) AG 50W-X12 0.2mol/L盐酸 双柱 [46] 11 石英柱
(柱高>20cm)AG 50W-X8 (15mL)、
AG 50W-X12 (5mL)0.2mol/L盐酸 双柱 [47] 12 Bio-rad聚丙烯柱
(柱高约7cm)AG 50W-X12
(1.0mL)两柱分别使用1mol/L硝酸、0.2mol/L硝酸 双柱 [31] 13 Bio-rad聚丙烯柱
(柱高分别为4cm、9cm)AG 50W-X8
(2.5mL、2.0mL)两柱分别使用0.2mol/L盐酸、0.5mol/L盐酸 双柱 [48] 14 聚丙烯柱+ Saville聚四氟乙烯柱
(柱高分别为20cm、18cm)AG 50W-X8
(6.4mL、2.3mL)0.5mol/L盐酸 双柱 [49] 15 聚丙烯柱+石英柱 AG 50W-X8
(1.2mL、1.5mL、1.0mL)三柱分别使用2.8mol/L盐酸、0.15mol/L盐酸和
0.15mol/L盐酸的30%乙醇混合溶液三柱 [39] 16 聚丙烯柱+石英柱 AG 50W-X8
(1.2mL、1.5mL、1.0mL)三柱分别使用4mol/L盐酸+2.8mol/L盐酸、0.15mol/L
盐酸和0.15mol/L盐酸的30%乙醇混合溶液三柱 [50] 17 聚丙烯柱和聚乙烯柱
(柱高分别为5.5cm、0.88cm)AG 50W-X12
(1mL、0.1mL)四柱分别使用2.8mol/L盐酸、0.15mol/L盐酸、0.5mol/L
盐酸的30%乙醇混合溶液和0.15mol/L盐酸四柱 [22] 18 双柱
(柱高分别为2cm和12.5cm)AG 50W-X12
(0.57mL、6.8mL)0.5mol/L盐酸 套柱 [51] 表 2 不同单柱法分离纯化锂过程中淋洗液类型、淋洗液用量及淋洗时间的对比
Table 2 Comparison of eluent type, eluent dosage and elution time during lithium separation and purification by different single column methods
表 3 不同双柱法分离纯化锂过程中淋洗液类型、淋洗液用量及淋洗时间的对比
Table 3 Comparison of eluent type, eluent dosage and eluent time during Li separation and purification by different dual-column methods
表 4 不同多柱法分离纯化锂过程中淋洗液类型、淋洗液用量及淋洗时间的对比
Table 4 Comparison of eluent type, eluent dosage and elution time during Li separation and purification by different multi-column methods
-
[1] Elliott T, Thomas A, Jeffcoate A, et al. Lithium isotope evidence for subduction-enriched mantle in the source of mid-ocean-ridge basalts[J]. Nature, 2006, 443(7111): 565−568. doi: 10.1038/nature05144
[2] Liu M C, McKeegan K D, Goswami J N, et al. Isotopic records in CM hibonites: Implications for timescales of mixing of isotope reservoirs in the Solar nebula[J]. Geochimica et Cosmochimica Acta, 2009, 73(17): 5051−5079. doi: 10.1016/j.gca.2009.02.039
[3] Huh Y, Chan L H, Edmond J M. Lithium isotopes as a probe of weathering processes: Orinoco River[J]. Earth and Planetary Science Letters, 2001, 194(1-2): 189−199. doi: 10.1016/S0012-821X(01)00523-4
[4] Misra S, Froelich P N. Lithium isotope history of cenozoic seawater: Changes in silicate weathering and reverse weathering[J]. Science, 2012, 335(6070): 818. doi: 10.1126/science.1214697
[5] Miao W L, Zhang X Y, Li Y L, et al. Lithium and strontium isotopic systematics in the Nalenggele River catchment of Qaidam Basin, China: Quantifying contributions to lithium brines and deciphering lithium behavior in hydrological processes[J]. Journal of Hydrology (Part B), 2022, 614: 128630. doi: 10.1016/j.jhydrol.2022.128630
[6] 刘丽君, 王登红, 侯可军, 等. 锂同位素在四川甲基卡新三号矿脉研究中的应用[J]. 地学前缘, 2017, 24(5): 167−171. doi: 10.13745/j.esf.yx.2017-1-16 Liu L J, Wang D H, Hou K J, et al. Application of lithium isotope to Jiajika new No. 3 pegmate lithium poly-metallic vein in Sichuan[J]. Earth Science Frontiers, 2017, 24(5): 167−171. doi: 10.13745/j.esf.yx.2017-1-16
[7] Choi H B, Ryu J S, Shin W J, et al. The impact of anthropogenic inputs on lithium content in river and tap water[J]. Nature Communications, 2019, 10(1): 5371. doi: 10.1038/s41467-019-13376-y
[8] Kaplan L, Wilzbach K E. Lithium isotope determination by neutron activation[J]. Analytical Chemistry, 1954, 26(11): 1797−1798. doi: 10.1021/AC60095A031
[9] Meier A L. Determination of lithium isotopes at natural abundance levels by atomic absorption spectrometry[J]. Analytical Chemistry, 1982, 54(13): 2158−2161. doi: 10.1021/ac00250a007
[10] Grégoire D C, Acheson B M, Taylor R P. Measurement of lithium isotope ratios by inductively coupled plasma mass spectrometry: Application to geological materials[J]. Journal of Analytical Atomic Spectrometry, 1996, 11(9): 765−772. doi: 10.1039/JA9961100765
[11] Chaussidon M, Robert F. 7Li/6Li and 11B/10B variations in chondrules from the Semarkona unequilibrated chondrite[J]. Earth and Planetary Science Letters, 1998, 164(3): 577−589. doi: 10.1016/S0012-821X(98)00250-7
[12] Chan L H. Lithium isotope analysis by thermal ionization mass spectrometry of lithium tetraborate[J]. Analytical Chemistry, 1987, 59(22): 2662−2665. doi: 10.1021/AC00149A007
[13] Moriguti T, Nakamura E. Precise lithium isotopic analysis by thermal ionization mass spectrometry using lithium phosphate as an ion source material[J]. Proceedings of the Japan Academy, 1993, 69(6): 123−128. doi: 10.2183/PJAB.69.123
[14] Tomascak P B, Carlson R W, Shirey S B. Accurate and precise determination of Li isotopic compositions by multi-collector sector ICP-MS[J]. Chemical Geology, 1999, 158(1): 145−154. doi: 10.1016/S0009-2541(99)00022-4
[15] Magna T, Wiechert U H, Halliday A N. Low-blank isotope ratio measurement of small samples of lithium using multiple-collector ICP-MS[J]. International Journal of Mass Spectrometry, 2004, 239(1): 67−76. doi: 10.1016/j.ijms.2004.09.008
[16] 唐清雨, 陈露, 田世洪, 等. 锂硼同位素MC-ICP-MS分析中的记忆效应研究[J]. 岩矿测试, 2024, 43(2): 201−212. doi: 10.15898/j.ykcs.202310260167 Tang Q Y, Chen L, Tian S H, et al. A study on memory effects in lithium and boron isotope analysis using MC-ICPMS[J]. Rock and Mineral Analysis, 2024, 43(2): 201−212. doi: 10.15898/j.ykcs.202310260167
[17] Bell D R, Hervig R L, Buseck P R, et al. Lithium isotope analysis of olivine by SIMS: Calibration of a matrix effect and application to magmatic phenocrysts[J]. Chemical Geology, 2009, 258(1): 5−16. doi: 10.1016/j.chemgeo.2008.10.008
[18] Martin C, Ponzevera E, Harlow G. In situ lithium and boron isotope determinations in mica, pyroxene, and serpentine by LA-MC-ICP-MS[J]. Chemical Geology, 2015, 412: 107−116. doi: 10.1016/j.chemgeo.2015.07.022
[19] Chan L H, Edmond J M. Variation of lithium isotope composition in the marine environment: A preliminary report[J]. Geochimica et Cosmochimica Acta, 1988, 52(6): 1711−1717. doi: 10.1016/0016-7037(88)90239-6
[20] Chan L H, Edmond J M, Thompson G, et al. Lithium isotopic composition of submarine basalts: Implications for the lithium cycle in the oceans[J]. Earth and Planetary Science Letters, 1992, 108(1): 151−160. doi: 10.1016/0012-821X(92)90067-6
[21] 孙爱德, 肖应凯. 锂同位素质谱法测定及其样品制备研究进展[J]. 盐湖研究, 2001, 9(4): 64−71. doi: 10.3969/j.issn.1008-858X.2001.04.011 Sun A D, Xiao Y K. The progress in the isotopic determination of lithium by TIMS and its sample preparation[J]. Journal of Salt Lake Research, 2001, 9(4): 64−71. doi: 10.3969/j.issn.1008-858X.2001.04.011
[22] Moriguti T, Nakamura E. High-yield lithium separation and the precise isotopic analysis for natural rock and aqueous samples[J]. Chemical Geology, 1998, 145(1): 91−104. doi: 10.1016/S0009-2541(97)00163-0
[23] Chan L H, Edmond J M, Thompson G. A lithium isotope study of hot springs and metabasalts from mid-ocean ridge hydrothermal systems[J]. Journal of Geophysical Research, 1993, 98(B6): 9653. doi: 10.1029/92JB00840
[24] Chan L H C, Gieskes J M, You C F, et al. Lithium isotope geochemistry of sediments and hydrothermal fluids of the Guaymas Basin, Gulf of California[J]. Geochimica et Cosmochimica Acta, 1994, 58(20): 4443−4454. doi: 10.1016/0016-7037(94)90346-8
[25] 肖应凯, 祁海平, 王蕴慧, 等. 青海大柴达木湖卤水、沉积物和水源水中的锂同位素组成[J]. 地球化学, 1994, 23(4): 329−338. doi: 10.19700/j.0379-1726.1994.04.003 Xiao Y K, Qi H P, Wang Y H, et al. Lithium isotopic composition of brine, sediments and source water in Da Qaidam Lake, Qinghai, China[J]. Geochimica, 1994, 23(4): 329−338. doi: 10.19700/j.0379-1726.1994.04.003
[26] 汪齐连. 运用MC-ICP-MS测定天然样品的锂同位素组成[D]. 北京: 中国科学院研究生院(地球化学研究所), 2006. Wang Q L. Measurement of lithium isotope in natural samples using MC-ICP-MS[D]. Beijing: Graduate School of Geochemistry, Chinese Academy of Sciences (Institute of Geochemistry), 2006.
[27] Millot R, Vigier N, Gaillardet J. Behaviour of lithium and its isotopes during weathering in the Mackenzie Basin, Canada[J]. Geochimica et Cosmochimica Acta, 2010, 74(14): 3897−3912. doi: 10.1016/j.gca.2010.04.025
[28] Penniston-Dorland S, Liu X M, Rudnick R L. Lithium isotope geochemistry[J]. Reviews in Mineralogy and Geochemistry, 2017, 82(1): 165−217. doi: 10.2138/rmg.2017.82.6
[29] Tomascak P, Magna T, Dohmen R. Advances in lithium isotope geochemistry[M]. Springer International Publishing, 2016.
[30] Hoefs J. Lithium isotope composition of Quaternary and Tertiary biogene carbonates and a global lithium isotope balance[J]. Geochimica et Cosmochimica Acta, 1997, 61(13): 2679−2690. doi: 10.1016/S0016-7037(97)00101-4
[31] Zhang X. Sedimentary recycling and chemical weathering: A silicon and lithium isotopes perspective[D]. Paris: Institut du Physique de Globe de Paris, 2018.
[32] 汪齐连, 赵志琦, 刘丛强, 等. 天然样品中锂的分离及其同位素比值的测定[J]. 分析化学, 2006, 34(6): 764−768. doi: 10.3321/j.issn:0253-3820.2006.06.005 Wang Q L, Zhao Z Q, Liu C Q, et al. Separation and determination of lithium in natural samples[J]. Chinese Journal of Analytical Chemistry, 2006, 34(6): 764−768. doi: 10.3321/j.issn:0253-3820.2006.06.005
[33] Bouman C, Elliott T, Vroon P Z. Lithium inputs to subduction zones[J]. Chemical Geology, 2004, 212(1): 59−79. doi: 10.1016/j.chemgeo.2004.08.004
[34] Dellinger M, Joshua West A, Paris G, et al. The Li isotope composition of marine biogenic carbonates: Patterns and mechanisms[J]. Geochimica et Cosmochimica Acta, 2018, 236: 315−335. doi: 10.1016/j.gca.2018.03.014
[35] Murphy J G, Ahm A S C, Swart P K, et al. Reconstructing the lithium isotopic composition (δ7Li) of seawater from shallow marine carbonate sediments[J]. Geochimica et Cosmochimica Acta, 2022, 337: 140−154. doi: 10.1016/j.gca.2022.09.019
[36] Yang C, Vigier N, Yang S, et al. Clay Li and Nd isotopes response to hydroclimate changes in the Changjiang (Yangtze) Basin over the past 14,000 years[J]. Earth and Planetary Science Letters, 2021, 561: 116793. doi: 10.1016/j.jpgl.2021.116793
[37] Schonbachler M, Fehr M A. Basic of ion exchange chromatography for selected geological applications[M].Treatise on Geochemistry (2nd Edition). 2014.
[38] 石雅静. 地质样品中钼同位素的自动化分离富集方法研究[D]. 成都: 成都理工大学, 2020. Shi Y J. Automatic separation and enrichment of molybdenum isotopes in geological samples[D]. Chengdu: Chengdu University of Technology, 2020.
[39] 苏嫒娜, 李真真, 田世洪, 等. MC-ICP-MS高精度测定Li同位素分析方法[J]. 矿床地质, 2010, 29(S1): 835−836. doi: 10.16111/j.0258-7106.2010.s1.393 Su Y N, Li Z Z, Tian S H, et al. High-precision measurement of lithium isotopes using MC-ICP-MS[J]. Mineral Deposits, 2010, 29(S1): 835−836. doi: 10.16111/j.0258-7106.2010.s1.393
[40] 蔺洁, 刘勇胜, 胡兆初, 等. MC-ICP-MS准确测定地质样品中锂同位素组成[J]. 矿物岩石地球化学通报, 2016, 35(3): 458−463. doi: 10.3969/j.issn.1007-2802.2016.03.008 Lin J, Liu Y S, Hu Z C, et al. Accurate analysis of lithium isotopic composition of geological samples by MC-ICP-MS[J]. Bulletin of Mineralogy, Petrology and Geochemistry, 2016, 35(3): 458−463. doi: 10.3969/j.issn.1007-2802.2016.03.008
[41] Nishio Y, Nakai S I. Accurate and precise lithium isotopic determinations of igneous rock samples using multi-collector inductively coupled plasma mass spectrometry[J]. Analytica Chimica Acta, 2002, 456(2): 271−281. doi: 10.1016/S0003-2670(02)00042-9
[42] Misra S, Froelich P N. Measurement of lithium isotope ratios by quadrupole-ICP-MS: Application to seawater and natural carbonates[J]. Journal of Analytical Atomic Spectrometry, 2009, 24(11): 1524−1533. doi: 10.1039/B907122A
[43] Lin J, Liu Y, Hu Z, et al. Accurate determination of lithium isotope ratios by MC-ICP-MS without strict matrix-matching by using a novel washing method[J]. Journal of Analytical Atomic Spectrometry, 2016, 31(2): 390−397. doi: 10.1039/C5JA00231A
[44] 张俊文, 孟俊伦, 赵志琦, 等. 多接收电感耦合等离子质谱法准确测定天然地质样品中的锂同位素组成[J]. 分析化学, 2019, 47(3): 415−422. doi: 10.19756/j.issn.0253-3820.181444 Zhang J W, Meng J L, Zhao Z Q, et al. Accurate determination of lithium isotopic compositions in geological samples by multi-collector inductively coupled plasma-mass spectrometry[J]. Chinese Journal of Analytical Chemistry, 2019, 47(3): 415−422. doi: 10.19756/j.issn.0253-3820.181444
[45] Zhu G, Ma J, Wei G, et al. A rapid and simple method for lithium purification and isotopic analysis of geological reference materials by MC-ICP-MS[J]. Frontiers in Chemistry, 2020, 8: 557489. doi: 10.3389/fchem.2020.557489
[46] James R H, Palmer M R. The lithium isotope composition of international rock standards[J]. Chemical Geology, 2000, 166(3): 319−326. doi: 10.1016/S0009-2541(99)00217-X
[47] Gao Y, Casey J F. Lithium isotope composition of ultramafic geological reference materials JP-1 and DTS-2[J]. Geostandards and Geoanalytical Research, 2012, 36(1): 75−81. doi: 10.1111/j.1751-908X.2011.00117.x
[48] Li W, Liu X M, Godfrey L V. Optimisation of lithium chromatography for isotopic analysis in geological reference materials by MC-ICP-MS[J]. Geostandards and Geoanalytical Research, 2019, 43(2): 261−276. doi: 10.1111/ggr.12254
[49] Li X, Han G, Zhang Q, et al. Accurate lithium isotopic analysis of twenty geological reference materials by multi-collector inductively coupled plasma mass spectrometry[J]. Spectrochimica Acta Part B: Atomic Spectroscopy, 2022, 188: 106348. doi: 10.1016/j.sab.2021.106348
[50] 赵悦, 侯可军, 田世洪, 等. 常用锂同位素地质标准物质的多接收器电感耦合等离子体质谱分析研究[J]. 岩矿测试, 2015, 34(1): 28−39. doi: 10.15898/j.cnki.11-2131/td.2015.01.006 Zhao Y, Hou K J, Tian S H, et al. Study on measurements of lithium isotopic compositions for common standard reference materials using multi-collector inductively coupled plasma-mass spectrometry[J]. Rock and Mineral Analysis, 2015, 34(1): 28−39. doi: 10.15898/j.cnki.11-2131/td.2015.01.006
[51] Zhu Z, Yang T, Zhu X. Achieving rapid analysis of Li isotopes in high-matrix and low-Li samples with MC-ICP-MS: New developments in sample preparation and mass bias behavior of Li in the ICPMS[J]. Journal of Analytical Atomic Spectrometry, 2019, 34(7): 1503−1513. doi: 10.1039/C9JA00076C
[52] Gou L F, Jin Z D, Pogge von Strandmann P A E, et al. Li isotopes in the middle Yellow River: Seasonal variability, sources and fractionation[J]. Geochimica et Cosmochimica Acta, 2019, 248: 88−108. doi: 10.1016/j.gca.2019.01.007
[53] Ma T, Weynell M, Li S L, et al. Lithium isotope compositions of the Yangtze River headwaters: Weathering in high-relief catchments[J]. Geochimica et Cosmochimica Acta, 2020, 280: 46−65. doi: 10.1016/j.gca.2020.03.029
[54] Zhu G, Ma J, Wei G, et al. Lithium isotope fractionation during the weathering of granite: Responses to pH[J]. Geochimica et Cosmochimica Acta, 2023, 345: 117−129. doi: 10.1016/j.gca.2022.12.028
[55] Alistair B, Jeffcoate T E, Thomas A, et al. Precise, small sample size determinations of lithium isotopic compositions of geological reference materials and modern seawater by MC-ICP-MS[J]. Geostandards and Geoanalytical Research, 2004, 28(1): 161−172. doi: 10.1111/j.1751-908X.2004.tb01053.x
[56] Bohlin M S, Misra S, Lloyd N, et al. High-precision determination of lithium and magnesium isotopes utilising single column separation and multi-collector inductively coupled plasma mass spectrometry[J]. Rapid Communications in Mass Spectrometry, 2018, 32(2): 93−104. doi: 10.1002/rcm.8020
[57] 宋以龙. 我国大型河流流域硅酸盐岩风化过程的锂同位素制约[D]. 天津: 天津大学, 2021. Song Y L. Continental silicate weathering processes traced by Li isotopes in Chinese large rivers[D]. Tianjin: Tianjin University, 2021.
[58] Rudnick R L, Tomascak P B, Njo H B, et al. Extreme lithium isotopic fractionation during continental weathering revealed in saprolites from South Carolina[J]. Chemical Geology, 2004, 212(1): 45−57. doi: 10.1016/j.chemgeo.2004.08.008
[59] 李超, 王登红, 屈文俊, 等. 关键金属元素分析测试技术方法应用进展[J]. 岩矿测试, 2020, 39(5): 658−669. doi: 10.15898/j.cnki.11-2131/td.201907310115 Li C, Wang D H, Qu W J, et al. A review and perspective on analytical methods of critical metal elements[J]. Rock and Mineral Analysis, 2020, 39(5): 658−669. doi: 10.15898/j.cnki.11-2131/td.201907310115



 下载:
下载:

 京公网安备 11010202008159号
京公网安备 11010202008159号