Research Progress in Analytical Methods of Biomarker GDGTs in Geological Environments
-
摘要:
甘油二烷基甘油四醚脂(GDGTs)是一类来自于微生物细胞膜脂的新兴生物标志物,广泛存在于海洋、湖泊、土壤、泥炭等环境。在活体细胞中,GDGTs通常以完整极性膜脂(IPL-GDGTs)的形式存在,而在地质环境中主要以脱去极性头基的核心脂(CL-GDGTs)的形式存在。CL-GDGTs结构稳定、不易降解,并且对环境变化响应敏感,因此被认为是重建古气候-古环境变化的有力工具。GDGTs结构复杂、种类多样,在环境中的含量通常较低且常与其他化合物共存,因此分析难度较高,现有技术和方法在其分离、纯化、定量等方面仍然面临挑战。本文总结了近年来GDGTs在分析技术方面的研究进展,概述了GDGTs的分类与结构,对环境中IPL-GDGTs和CL-GDGTs的分离、纯化等方法进行总结和比较,其中CL-GDGTs可选择多种提取方法,而极性较强、热稳定性较差的IPL-GDGTs应尽量选取Bligh-Dyer提取法。普通的分离、纯化通常采用柱层析法,而涉及GDGTs单体分离时,一般采用制备液相色谱法。液相色谱-质谱、核磁共振波谱、气相色谱-同位素比值质谱是GDGTs含量测定、结构鉴定、同位素分析的主要分析手段。本文评述了现有方法的特点和不足,并在此基础上,提出了GDGTs分析技术的发展方向,以期为地质环境中GDGTs的分析研究提供启示和参考。
 要点
要点(1)GDGTs是来自微生物细胞膜脂的一类新兴的生物标志物,是当前古气候-古环境重建研究的良好载体和有力工具,在地质环境中广泛分布。
(2)GDGTs的结构复杂、种类多样,分析难度较高,现有分析技术在其分离、纯化、准确定量等方面仍面临挑战。
(3)GDGTs的分析研究重点需要关注方法标准化、分析效率、新组分识别及同位素分析等方面。
HIGHLIGHTS(1) GDGTs are a new class of biomarkers, which are ubiquitous in geological environments and have unique advantages in paleoclimate reconstruction.
(2) The analysis of GDGTs is difficult due to their structural diversity, and the existing analytical methods still face challenges in separation, purification, and accurate quantification.
(3) The future analysis of GDGTs should focus on improving the analytical separation, efficiency, and accuracy and expand to method standardization, new component identification, and isotopic techniques.
Abstract:Glycerol dialkyl glyceryl tetraethers (GDGTs) are a class of environment biomarkers that are widely found in the environment of oceans, lakes, soils, and peat. GDGTs usually exist as intact polar lipids (IPL-GDGTs) in living cells, while they exist as core lipids stripped of polar head groups (CL-GDGTs) in geological environments. CL-GDGTs are structurally stable and sensitive to environmental changes and are considered to be a powerful tool for reconstructing palaeoclimate-palaeoenvironmental changes. GDGTs are structurally complex and diverse, coexisting with other compounds and present low contents, which brings challenges in analysis, especially in separation, purification, and quantification. This article summarizes the classification and structure of GDGTs, and presents a summary and comparison of methods for the separation and purification of IPL-GDGTs and CL-GDGTs in the environment. Multiple extraction methods can be used for CL-GDGTs, while the polar and thermally unstable IPL-GDGTs are preferably extracted using the Bligh-Dyer method. This article reviews the characteristics and limitations of various analysis methods, including liquid chromatography-mass spectrometry, nuclear magnetic resonance spectroscopy, and gas chromatography-isotope ratio mass spectrometry. The BRIEF REPORT is available for this paper at http://www.ykcs.ac.cn/en/article/doi/10.15898/j.ykcs.202306100077.
-
Keywords:
- glycerol dialkyl glycerol tetraethers (GDGTs) /
- extraction /
- separation and purification /
- content analysis /
- structural identification /
- isotopic analysis
 BRIEF REPORT
BRIEF REPORTGlycerol dialkyl glycerol tetraethers (GDGTs) are lipid biomarkers that are widely distributed in geological environments, including oceans[4-6], lakes[7-10], river estuaries[11], hot springs[12], soil[14-17], and peats[18-22]. They are sensitive to environmental changes and can effectively record paleoclimate information over a range of geological time scales. GDGT-based proxies are widely used in the reconstruction of terrestrial and marine paleoenvironments[23-28]. However, the accurate analysis of GDGTs is challenging due to their diverse nature and difficulties in their separation, purification, and quantification.
GDGTs in the environment exist in two forms: intact polar lipids (IPL-GDGTs) or as core lipids (CL-GDGTs) (Fig.1). CL-GDGTs can be further classified into two types based on their structural differences: isoprenoid GDGTs (isoGDGTs) and branched GDGTs (brGDGTs). IsoGDGTs are primarily composed of GDGT-0 to GDGT-8, Crenarchaeol, and its stereoisomer. IsoGDGTs are produced by archaea[36-37], while the biological origin of brGDGTs is still uncertain, although there is evidence to indicate that their potential producers are heterotrophic bacteria, including Acidobacteria, Proteobacteria, Nitrospira, Bacteroidetes, Actinobacteria, and Verrucomicrobia[25-27, 48]. BrGDGTs consist of three series (Ⅰ, Ⅱ, and Ⅲ), each of which differs by a methylene group. The alkyl side chains of brGDGTs in series Ⅱ and Ⅲ may possess various methyl groups at the C5, C6, and C7 positions, leading to the formation of positional isomers, such as 5-methyl, 6-methyl, and 7-methyl brGDGTs[8, 39-41].
Extraction methods for GDGTs from environmental samples mainly involve the Bligh-Dyer method[12, 35, 49-50], ultrasonic extraction[14, 32, 51-52], Soxhlet extraction[53-56], accelerated solvent extraction (ASE)[31, 57-59] and microwave-assisted extraction (MAE)[27, 59-60]. The Bligh-Dyer method was originally used to extract cell membrane lipids from eukaryotes and bacteria, but modifications have been made to increase extraction rates[3, 59, 61]. Ultrasonic extraction usually uses methanol, methanol-dichloromethane, and dichloromethane as the extraction solution[52, 59]. Soxhlet extraction is highly efficient but demands a large amount of solvents and is time-consuming[53-56]. ASE is the most commonly used method for extracting CL-GDGTs, using dichloromethane-methanol as the extraction solvent[57-59]. MAE is a simple, fast, efficient, and selective method for solid samples, but its application is limited by the lack of widespread microwave reactor availability[28, 59-60].
Different GDGTs extraction methods have been compared in several studies[50-51, 63-64]. Schouten et al.[51] compared the efficiency of three extraction methods: ultrasonic extraction, Soxhlet extraction, and ASE extraction for isoGDGTs. They found that the TEX86 values obtained by the three methods were almost identical within ±1σ error. Wang et al[50] also compared the efficiency of Bligh-Dyer and ultrasonic extraction. They found that the Bligh-Dyer method was more effective in extracting IPL-GDGTs, whereas the ultrasonic extraction method was more efficient in extracting CL-GDGTs. However, the values of palaeoclimatic indicators (TEX86, MBT, and CBT) obtained by both methods were identical, indicating that the different extraction methods did not affect the value of palaeoclimatic proxies[50]. Yang et al.[64] later confirmed that the Bligh-Dyer method was more efficient than the ultrasonic extraction in extracting OH-GDGTs that contain polar head groups. In summary, the Bligh-Dyer method is preferable for IPL-GDGTs due to their greater polarity and lower thermal stability, while various extraction methods can be used for CL-GDGTs.
To isolate and purify GDGTs from environmental samples, column chromatography[51, 59-61] is commonly used along with preparative liquid chromatography (Prep HPLC) in some cases[40, 65-66]. Prep HPLC is mainly used to separate components that are hard to fractionate or prone to loss in column chromatography, such as certain GDGT monomers, isomers, or IPL-GDGTs with unstable head groups[22, 39, 66, 68]. The Ib and IIIa5, 6 components were successfully isolated from mixtures of brGDGTs through Prep HPLC along with repeated separation and purification[18, 40]. Silica and alumina (Al2O3) are commonly used as stationary phases. The elution process yields nonpolar and polar fractions. GDGTs are usually found in the polar fraction and can be further analysed for structural identification and isotopic analysis using Prep HPLC or a combination of both methods. During the process of separating and purifying GDGTs, some IPL-GDGTs may lose their polar head groups due to poor stability. To address this issue, Huguet et al.[59] proposed an indirect method for determining IPL-GDGTs. The total lipid extract was divided into two equal parts. The first part was purified through an Al2O3 column to obtain the content of CL-GDGTs. The second part underwent acid-hydrolysis, which converted IPL-GDGTs into CL-GDGTs by losing their polar head groups. The hydrolysis product was the sum of (IPL+CL)-GDGTs. The quantity of IPL-GDGTs was determined by subtracting CL-GDGTs from (IPL+CL)-GDGTs. However, the error of this “ subtraction method” is greater than that of the direct measurement. Therefore, this method should be used with caution, especially when the concentration of IPL-GDGTs is lower than that of CL-GDGTs[59].
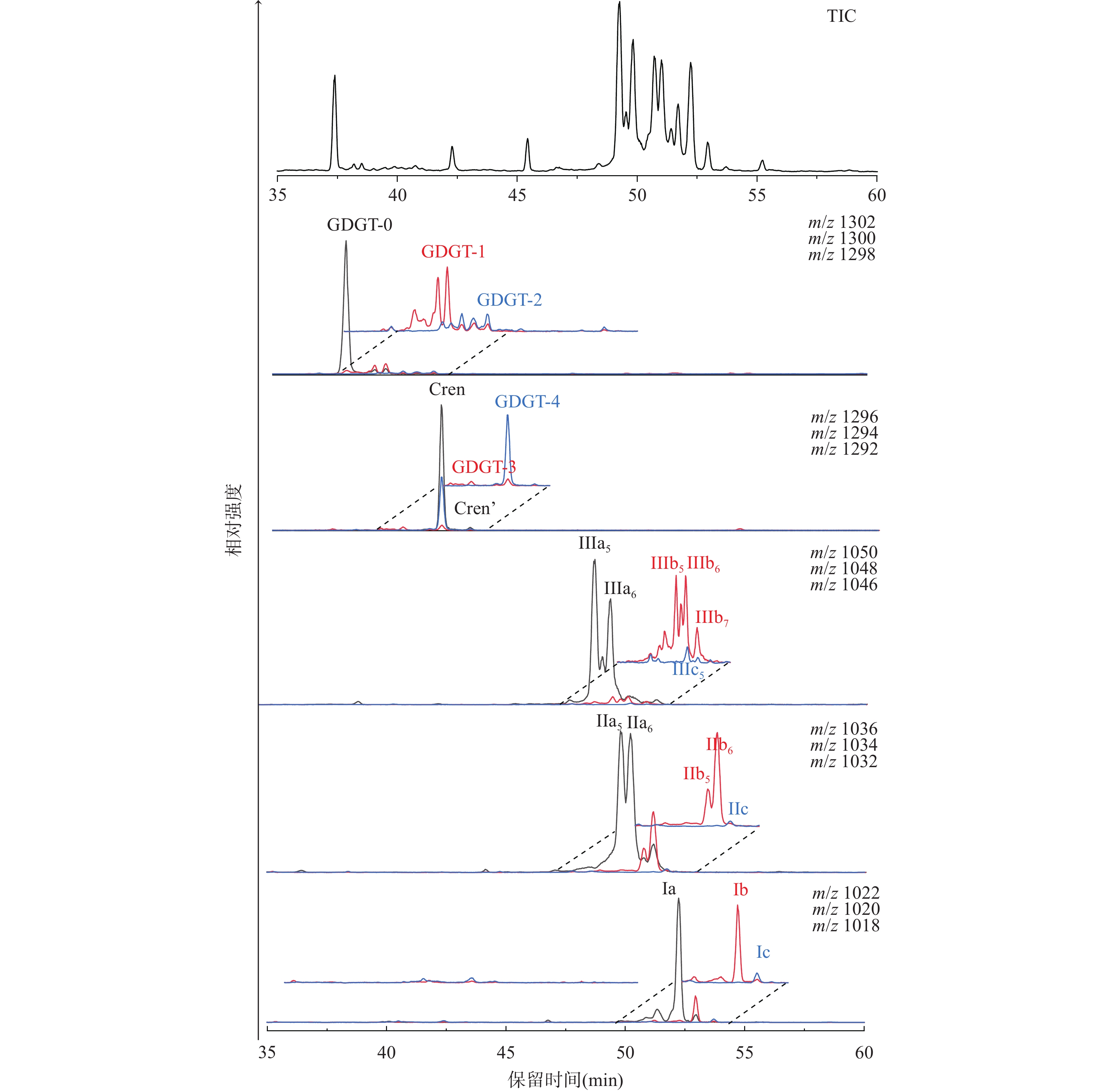
The analysis of GDGTs in the geological environment mainly involves content determination, structural identification, and isotopic analysis. This is commonly done using high-performance liquid chromatography-mass spectrometry (HPLC-MS)[3, 51-54], gas chromatography-mass spectrometry (GC-MS)[3, 63, 69-70], nuclear magnetic resonance spectroscopy (NMR)[18, 41, 71], and gas chromatography-isotope ratio mass spectrometry (GC-IRMS)[40, 65, 72]. CL-GDGTs are usually analyzed by HPLC-APCI-MS, while IPLs-GDGTs with polar head groups are usually analyzed by RP-HPLC-ESI-MS. Good separation of 5-, 6-, and 7-methyl isomers of brGDGTs can be achieved by using four HPLC columns or two UPLC columns in tandem[8, 39-41, 73]. In a normal-phase HPLC system, the peaks of GDGTs are sorted by their mass-to-charge ratios (m/z) from the largest to the smallest, as shown in Fig. 2.
Single and triple quadrupole mass spectrometry are commonly used for the GDGTs analysis through HPLC-MS. SIM mode is preferred for quantification due to its higher sensitivity and reproducibility than the full-scan mode, which captures the characteristic ions [M+H]+ of each GDGT component[51]. However, low resolution can cause ion loss for isoGDGTs and brGDGTs, which affects the accuracy of quantitative results[76]. Recently, high-resolution mass spectrometry, such as Orbitrap-MS[77-78] and FTICR-MS[55, 79], has been applied to the GDGTs analysis. HPLC-HRMS provides a comprehensive characterization of the structure and composition of GDGTs in environmental samples, offering great potential for future analyses.
Structure identification of GDGTs involves various techniques such as HRMS, GC-MS, and NMR techniques[18, 42, 63, 70]. The molecular formulae of GDGTs can be determined by HRMS through precise mass measurement of the protonated molecular ion peaks and isotopic peaks evaluation[77, 79]. Conventional GC has a temperature limit of 300-350°C, which makes it impossible to vaporize GDGTs directly. However, HT-GC has a higher limit of 400-450°C, allowing for direct analysis of GDGTs. HT-GC equipped with a flame ionization detector can be used to analyse CL-GDGTs[18, 72]. The possible molecular structures of GDGTs can be deduced from analyzing the primary and secondary fragment ions of the mass spectra, along with their fragmentation pathways[41-42]. NMR techniques provide molecular structural information of GDGTs, which can be verified by other methods such as HPLC-MS and GC-MS[18, 41, 71, 82]. Nevertheless, NMR has certain limitations and requires high sample purity and content to ensure adequate signal intensity. NMR analysis usually requires high sample purity and milligram injection quantities, which means it is not easy to separate and enrich GDGT monomers from their homologues and isomers, so only a few GDGT components (e.g., Ia, Ib, and IIa) have been verified by NMR[18, 44, 71]. In the future, HPLC-NMR technology may simplify the process and achieve more efficient and convenient structural analysis of GDGTs[84].
The isotopic composition of GDGTs provides vital biological and environmental information [65, 85-90]. GC-IRMS is the primary method used for stable carbon and hydrogen isotope analysis of GDGTs[40, 61, 65, 87-89]. Prior to GC-IRMS analysis, GDGTs are converted into smaller and more volatile alkanes through “ether bond cleavage”[61, 69-70, 85]. Accelerator mass spectrometry is used to analyze radiocarbon isotopes for GDGTs, but it requires a carbon content of the sample greater than 100μg C[93]. To meet this testing requirements, a large amount of environmental samples must be extracted, separated, and purified to obtain an adequate amount of high-purity individual GDGT component[18, 90].
Significant progress has been achieved in the analytical techniques and methods for GDGTs during the last two decades. However, existing approaches still face issues regarding standardization, method efficiency, and novel GDGT components. Future studies should focus on the following aspects: (1) Establishing standard methods and quality control systems for GDGTs. Developing standard analytical procedures for GDGTs and establishing a quality control and evaluation system is crucial to improve the reliability and comparability of the analytical results. (2) Developing convenient and efficient analytical methods. It is necessary to establish simpler and more automated techniques for GDGT extraction, separation, and purification. In addition, developing qualitative and quantitative methods with better sensitivity and accuracy is also important. (3) Identification of unknown components of GDGTs. Using various techniques such as HPLC-HRMS, GC-MS, and NMR to identify the unknown GDGT components, enhancing the understanding of their composition and distribution in the environment. (4) Developing new isotopic techniques and methods for GDGTs with fewer samples and easier procedures, and helping to expand the use of isotopic methods in the future.
-
生物标志物,是从生物体中演化而来的一系列有机分子,其含量、分布特征及同位素组成能够反映地质历史时期的气候和环境变化[1-2]。甘油二烷基甘油四醚脂(Glycerol dialkyl glyceryl tetraethers,简称GDGTs)是古菌或细菌细胞膜的骨架成分,也是二十世纪末发展起来的一类新兴的生物标志物[3]。GDGTs在自然界广泛分布,包括海洋[4-6]、湖泊[7-10]、河流[11]、热泉[12-13]、土壤[14-17]、泥炭[18-22]等环境。GDGTs的组成对外界环境变化响应敏感,并且其核心脂结构稳定、不易降解,能够长期保存在沉积环境中,因此被认为是古气候重建研究的良好载体和有力工具[23-28]。
目前,GDGTs已被应用于重建早白垩纪以来的气候环境演变历史[29-30]。例如,Chu等[31]利用湖光岩湖泊沉积物中GDGTs重建了末次冰消期以来中国热带地区温度变化,该结果与格陵兰冰芯记录的温度变化趋势大致相同,证实高纬度冰盖与热带陆地之间存在耦合作用;Bai等[32]利用土壤中GDGTs指标重建了青藏高原南部古海拔变化历史,为揭示印度板块与欧亚大陆板块的碰撞过程和机制提供了新线索。大量研究表明GDGTs在古气候重建中发挥了重要作用,而进行古气候重建的前提是准确分析GDGTs[29-32]。然而,GDGTs分子结构复杂,其同系物、异构体理化性质相似,并且环境中GDGTs的含量较低(通常在ng/g水平),又常与其他有机化合物共存,因此分析难度较高,现有技术在其分离、纯化、准确定量等方面仍面临挑战[1,3]。
本文在前人研究基础上,概述了GDGTs的结构与分类,总结并比较了地质环境样品中GDGTs的提取、分离、纯化等前处理方法,评述了液相色谱-质谱、核磁共振波谱、气相色谱-同位素比值质谱等技术在含量分析、结构鉴定、同位素分析方面的研究进展,同时提出未来GDGTs分析技术的发展方向。
1. GDGTs的结构与分类
环境中的GDGTs种类多样,通常所说的GDGTs是指微生物的核心脂(Core Lipids,CL-GDGTs),其基本结构包括两个烷基长链和两个甘油分子,烷基长链末端通过醚键与甘油基团结合形成闭合环状大分子[33-34]。在活体细胞中,CL-GDGTs两端的甘油基团上带有由磷酸盐、醣基或葡萄糖醛酸等组成的极性头基,以完整极性膜类脂(Intact Polar Lipids,IPL- GDGTs)的形式存在(图1),但随着细胞的死亡降解,IPL-GDGTs会丢失极性头基而转化成CL-GDGTs[16,18,35]。
目前,研究较多的CL-GDGTs主要包括两大类:类异戊二烯GDGTs(Isopernoidal GDGTs,简称isoGDGTs)和支链GDGTs(Branched GDGTs,简称brGDGTs)。两者的结构既有相似性,又有明显差异(图1)。相同点在于:两者均是由2条长链烷基和2个甘油分子形成的四醚类环状化合物,并且烷基侧链上均含有一定数量的甲基和五元环或六元环。不同点在于:①两者结构中甘油的立体构型不同,isoGDGTs为甘油-1-磷酸(G-1-P)构型,而brGDGTs为甘油-3-磷酸(G-3-P)构型;②两者烷基侧链的骨架不同,isoGDGTs的烷基侧链含有40个碳原子且为类异戊二烯结构[12],而brGDGTs的侧链仅由28个碳原子组成且为支链烷烃结构[6](图1)。
isoGDGTs主要包括GDGT-0到GDGT-8(数字代表其结构中包含的环戊烷数量)、泉古菌醇(Crenarchaeol,简称Cren)及其立体异构体(Crenarchaeol regioisomer,简称Cren’)(图1)[12]。isoGDGTs的母源微生物是古菌,包括泉古菌(Crenarchaeota)、奇古菌(Thaumarchaea)、广古门菌(Euryarchaeota)等[36-37]。其中,泉古菌主要合成含有0~4个环戊烷的GDGTs和Cren,氨氧化古菌主要合成Cren及Cren’,而含有4个环戊烷以上的isoGDGTs(GDGT-5至GDGT-8)仅在热泉和嗜热菌的培养液中发现[38]。
brGDGTs含有Ⅰ、Ⅱ和Ⅲ三个系列,每个系列之间相差一个CH2。目前已发现Ⅱ和Ⅲ系列烷基侧链上的甲基可以在C5、C6、C7位置变动,分别形成5-甲基、6-甲基和7-甲基等位置异构体[8,39-41]。在立体结构方面,brGDGTs的甘油构型只有一种反平行结构[42],这与Cren及Cren’存在平行和反平行2种构型不同[43],并且其烷基侧链上的多个手性碳原子的立体构型也是固定的[44]。目前,brGDGTs的生物来源还不十分明确,但其甲基支链结构和甘油立体化学特征指示它们由细菌合成[8],其稳定碳、氢同位素表明其来自兼性异养细菌[21,45]。培养实验也证实酸杆菌可以合成brGDGTs个别组分(如Ⅰa、Ⅰc)[46-47]。最新研究表明,除了酸杆菌外,变形菌、消化螺旋菌、拟杆菌、放线菌和疣菌也可能是brGDGTs的潜在母源[25-27,48]。
2. 地质环境样品中GDGTs的提取方法与纯化技术
2.1 GDGTs的提取方法
地质环境样品中GDGTs的提取方法主要包括Bligh-Dyer法(简称BD法)[12,35,49-50]、超声萃取法[14,32,51-52]、索氏抽提法[53-56]、加速溶剂萃取法(ASE)[31,57-59]和微波辅助提取法(MAE)[21,59-60]等(表1)。
表 1 地质环境样品中GDGTs的不同提取、分离、纯化方法Table 1. Different extraction, separation and purification methods of GDGTs in geological environment samples.样品类型 提取方法及提取剂 分离及净化方法 参考文献 土壤 BD法,甲醇-二氯甲烷-磷酸缓冲液 硅胶柱,正己烷/乙酸乙酯、乙酸乙酯、甲醇 Pitcher等(2009) 土壤 超声萃取法,甲醇、甲醇-二氯甲烷 硅胶柱,正己烷/二氯甲烷、二氯甲烷/甲醇 Bai等(2017) 土壤、湖泊沉积物 BD法,甲醇-二氯甲烷-磷酸缓冲液 硅胶柱,正己烷/乙酸乙酯、甲醇 Buckles等(2014) 土壤、湖泊沉积物 索氏抽提法,甲醇-二氯甲烷 Al2O3柱,正己烷/二氯甲烷、二氯甲烷/甲醇 Hu等(2016) 黄土 ASE,二氯甲烷-甲醇 硅胶柱,二氯甲烷/乙酸乙酯 Lu等(2016) 泥炭 超声萃取法,二氯甲烷、二氯甲烷/甲醇 硅胶柱,正己烷/甲醇 Zheng等(2018) 泥炭 MAE,二氯甲烷-甲醇 硅胶柱,二氯甲烷/甲醇 Naafs等(2017) 海洋沉积物 索氏抽提法,甲醇-二氯甲烷 Al2O3柱,正己烷/二氯甲烷、二氯甲烷/甲醇 Liao等(2020) 海洋沉积物 BD法,甲醇-二氯甲烷-磷酸缓冲液 硅胶柱,正己烷/乙酸乙酯
制备HPLC,正己烷/异丙醇Zhu等(2013) 湖泊沉积物 ASE法,二氯甲烷-甲醇 Al2O3柱,二氯甲烷、二氯甲烷/甲醇 Chu等(2017) 湖泊沉积物 ASE法,二氯甲烷-甲醇 Al2O3柱,正己烷/二氯甲烷、二氯甲烷/甲醇 Li等(2023) 湖泊沉积物 ASE法,二氯甲烷-甲醇 Al2O3柱,二氯甲烷、二氯甲烷/甲醇
制备HPLC,正己烷/异丙醇Weber等(2015 海洋、湖泊沉积物 MAE法,二氯甲烷-甲醇 Al2O3柱,正己烷/二氯甲烷、二氯甲烷/甲醇 Escala等(2009) 湖泊水体悬浮颗粒物 BD法,甲醇-二氯甲烷-磷酸缓冲液 Al2O3柱,正己烷/二氯甲烷、二氯甲烷/甲醇 Kumar等(2019) BD法最早被应用于提取真核生物和细菌中的细胞膜脂,以氯仿-甲醇(2∶1,V/V)为提取液,后经不断改良,如增加萃取液中甲醇的比例,加入少量的酸(如盐酸、三氯乙酸、磷酸盐缓冲液),使其对脂类物质的提取率可达90%以上[3,59,61]。超声波萃取法通常采用甲醇、甲醇-二氯甲烷(1∶1,V/V)、二氯甲烷等为提取液,超声萃取2~3次以保证提取效率[52,59]。索氏抽提法通常采用二氯甲烷-甲醇混合溶剂为萃取液,加热回流提取24~72h,其萃取效率较高,但溶剂消耗量大且耗时、费力[53-56]。ASE法是目前CL-GDGTs的主流提取方法,通常以二氯甲烷-甲醇为提取溶剂,在温度100~120℃、压力1200~1500psi条件下,静态萃取5~10min,循环萃取2~3次[57-59]。2020年Auderset等[62]提出一种ASE分步提取法,通过不同极性溶剂萃取,从土壤和沉积物中依次分离出正构烷烃、长链烯酮及GDGTs三种组分。MAE法是一种利用微波加热技术对固体样品提取的方法,通常以二氯甲烷-甲醇为提取溶剂,在一定温度、压力和微波辐射下,萃取10~20min,该法操作简单、快速高效、节省溶剂、选择性好,但由于微波反应器的普及程度不高,所以目前应用还较少[28,59-60]。
不同方法对GDGTs的提取效率不尽相同[50-51,63-64]。Schouten等[51]比较了超声萃取、索氏抽提和ASE萃取三种方法对isoGDGTs的提取效率,发现三种方法得到的古气候指标(TEX86)值在±1σ误差范围内基本一致。Huguet等[59]比较了BD法、超声法、索氏抽提法、ASE、MAE等多种提取方法,认为索氏提取法对于IPL-GDGTs的提取效果最佳,优于常用的BD法。随后,Lengger等[63]比较了BD法、索氏抽提法和ASE法,发现三种提取方法对CL-GDGTs的提取效率基本一致且良好,但对IPL-GDGTs的提取效率则呈现:ASE法<索氏抽提法<BD法,其原因可能是较高的温度(ASE法100℃,索氏抽提法65℃)导致部分IPL-GDGTs降解,而常温下进行的BD法更好地保护了IPL-GDGTs的极性头基。王欢业等[50]对比了BD法和超声萃取法的提取效率,指出BD法对于IPL-GDGTs的提取效果更好,而超声萃取法对于CL-GDGTs的提取效率更高,但两种方法得到的古气候指标(TEX86、MBT、CBT)数值一致,说明不同提取方法并不影响古气候指标的计算。之后,Yang等[64]也证实对于含有极性头基的OH-GDGTs,使用BD法比超声萃取法的提取效率更高。综上可知,CL-GDGTs的提取可以选择多种方法,而对于极性较强、热稳定性较差的IPL-GDGTs,应尽量选取BD法。
2.2 GDGTs的分离与纯化方法
在得到总脂类提取物后,需要从中进一步分离、纯化GDGTs,这个过程通常采用柱层析法[51,59-61],少数情况下采用制备液相色谱法[40,65-66]。前者适合常规的含量分析,后者或两者联用适合GDGTs的结构鉴定和同位素分析。柱层析法通常采用硅胶、氧化铝(Al2O3)等吸附剂作为固定相填料[51,59-61]。Al2O3作为固定相时,通常以正己烷-二氯甲烷、甲醇-二氯甲烷为淋洗液[31,51,57]。硅胶作为固定相时,通常以正己烷-乙酸乙酯、二氯甲烷-乙酸乙酯等为洗脱液[58,66]。收集到的洗脱液通常包括非极性和极性两部分,其中GDGTs存在于极性组分,将其用氮气吹干,溶解于合适的溶剂,过滤后即可上机分析[3,40]。柱层析色谱法的操作性强、分离效果好,但操作繁琐、溶剂消耗量大、容易产生误差。Sanchi等[67]提出一种采用固相萃取装置进行自动分离、净化的方法,使操作更简单、误差更小、重现性更高。
对于IPL-GDGTs,其含有的某些极性头基在硅胶柱净化、无水硫酸钠干燥、受热等过程中极易脱落[63]。为了避免IPL-GDGTs在分离、净化过程中损失,Huguet等[59]提出一种间接测定IPL-GDGTs的方法:首先把总脂类提取物平均分为两份,一份用Al2O3柱分离、净化,得到CL-GDGTs的含量;另一份在酸性条件下水解,使IPL-GDGTs丢失极性头基转化为CL-GDGTs,由此得到的水解产物即是(IPL+CL)-GDGTs的总和。最后,用(IPL+CL)- GDGTs减去CL-GDGTs,差值即为IPL-GDGTs含量。但需注意这种“差减法”的误差比直接测量法大,尤其当IPL-GDGTs的浓度比CL-GDGTs低时应谨慎使用[59]。
制备液相色谱法主要用于分离在柱层析法中不易分离或易损失的组分,如某些GDGTs单体、异构体或带有不稳定头基的IPL-GDGTs[22,39,66,68]。Weijers等[18]和Weber等[40]利用氨基制备色谱柱,通过制备液相色谱反复分离、纯化,从brGDGTs混合物中分离出高纯度、高浓度的Ib和IIIa5,6组分。Zhu等[66]采用两根极性正交的半制备色谱柱(一根分离正相体系的二醇基柱,一根分离反相体系的C18柱),通过制备液相色谱,从总脂类提取物中成功分离出2种带有糖苷头基的IPL-GDGTs。
3. 地质环境样品中GDGTs的分析技术
地质环境中GDGTs的分析主要涉及含量分析、结构鉴定、同位素分析三方面,相关分析主要借助于高效液相色谱-质谱仪(HPLC-MS)[3,51-54]、气相色谱-质谱仪[3,63,69-70]、核磁共振波谱仪[18,41,71]和气相色谱-同位素比值质谱仪[40,65,72]。
3.1 GDGTs含量分析
CL-GDGTs的分析通常采用高效液相色谱-大气压化学电离源-质谱法(HPLC-APCI-MS)。色谱柱一般选择氨基柱、氰基柱、硅胶柱等正相色谱柱,流动相一般选择正己烷-异丙醇[51,73]或者正己烷-乙酸乙酯[55,74]。采用2根UPLC色谱柱或4根HPLC色谱柱串联,可以使brGDGTs的同分异构体(如Ⅲa5、Ⅲa5,6、Ⅲa6与Ⅲa7)达到较好的分离效果[8,39-41,73]。在正相液相色谱体系下,GDGTs各组分的出峰顺序按照其质荷比(m/z)从大到小依次排列(图2)。isoGDGTs的[M+H]+范围在m/z 1286~1304之间,出峰顺序通常为:GDGT-0>GDGT-1> GDGT-2>GDGT-3>GDGT-4=Cren>Cren’。brGDGTs的[M+H]+范围在m/z 1018~1050之间,3个系列的整体出峰顺序为:Ⅲ>Ⅱ>Ⅰ,系列内的出峰顺序为:Ⅲa>Ⅲb>Ⅲc,IIa>IIb>IIc,Ia>Ib>Ic,但相邻系列组分的出峰顺序有时存在交叉的情况[8]。同分异构体(如Ⅲa、Ⅲb)的出峰顺序与其烷基侧链上甲基的位置有关,通常为:C5>C6>C7(图2)。
![]() 图 2 内蒙古双沟山天池湖泊沉积物中GDGTs的液相色谱总离子流图和提取离子流图图中分析条件:在正己烷-异丙醇梯度洗脱下,以2根BEH Hilic色谱柱(150mm×2.1mm×1.7μm)和1根硅胶色谱柱(150mm×2.1mm×1.9μm)串联,经HPLC-APCI-MS分析,实现GDGTs各组分良好分离。Figure 2. Total ion chromatogram (TIC) and extracted ion chromatogram (EIC) of GDGTs identified in lake sediment of Lake Shuanggoushan, Inner Mongolia. The chromatogram generated by HPLC-APCI-MS showing the elution order of brGDGTs and isoGDGTs with [M+H]+ ions.Analytical condition: under gradient elution of n-hexane/isopropanol, the good separation of GDGTs were achieved by HPLC-APCI-MS method with two BEH Hilic columns (150mm×2.1mm×1.7μm) and one silica column (150mm×2.1mm×1.9μm) in tandem.
图 2 内蒙古双沟山天池湖泊沉积物中GDGTs的液相色谱总离子流图和提取离子流图图中分析条件:在正己烷-异丙醇梯度洗脱下,以2根BEH Hilic色谱柱(150mm×2.1mm×1.7μm)和1根硅胶色谱柱(150mm×2.1mm×1.9μm)串联,经HPLC-APCI-MS分析,实现GDGTs各组分良好分离。Figure 2. Total ion chromatogram (TIC) and extracted ion chromatogram (EIC) of GDGTs identified in lake sediment of Lake Shuanggoushan, Inner Mongolia. The chromatogram generated by HPLC-APCI-MS showing the elution order of brGDGTs and isoGDGTs with [M+H]+ ions.Analytical condition: under gradient elution of n-hexane/isopropanol, the good separation of GDGTs were achieved by HPLC-APCI-MS method with two BEH Hilic columns (150mm×2.1mm×1.7μm) and one silica column (150mm×2.1mm×1.9μm) in tandem.IPLs-GDGTs带有极性头基,通常采用反相液相色谱(RP-HPLC)-电喷雾离子源-质谱仪(ESI-MS)分析[59,66,75]。Zhu等[66]运用反相超高效液相色谱-四极杆-飞行时间串联质谱,建立了一种可同时分析IPL-GDGTs和CL-GDGTs的方法,该方法比传统的正相方法(HPLC-APCI-MS)的灵敏度更高、色谱峰形更尖锐,并且能够得到与之一致的古气候指标值。在该方法基础上,Chen等[75]将RP-HPLC-ESI-MS与多反应监测(MRM)技术结合,建立了一种更适合分析IPL-GDGTs的方法,其采用的MRM模式比常用的选择离子扫描(SIM)模式具有更好的特异性和灵敏度,提高了方法的准确度、稳定性及重现性,并且检出限更低。
在质谱方面,单四极杆质谱和三重四极杆质谱是当前GDGTs分析最常用的质谱检测器。定量分析通常在SIM模式下进行,该模式通过采集GDGTs各组分的特征离子[M+H]+,使其灵敏度和重现性比全扫模式更好[51]。但由于四极杆质谱的分辨率不高,SIM模式会导致isoGDGTs和brGDGTs产生不同程度(18%~36%)的离子损失,在一定程度上降低了定量结果及古气候指标的准确性[76]。近年来,功能强大的高分辨率质谱也开始应用于GDGTs的分析,如静电场轨道阱质谱(Orbitrap-MS)[77-78]、傅里叶变换离子回旋共振质谱(FTICR-MS)[55,79]。高分辨质谱与HPLC联用,可以实现样品中GDGTs结构和组成的全面表征,并有助于GDGTs未知组分的鉴定。在离子源方面,CL-GDGTs极性较低,所以通常采用常压化学电离子源(APCI)。与电喷雾源(ESI)相比,APCI源的离子产率更高、准分子离子峰的强度更高[3,51]。近年来,也报道了采用大气压光致电离(APPI源)成果[77,79]。APPI源是一种新电离技术,其电离原理与APCI源相似,但其电离能量更低,对弱极性的CL-GDGTs的电离效果比APCI源更好,因此在未来分析中有很大潜力[77,79]。
目前,GDGTs定量分析主要采用内标法,通常以GTGT-C46作为内标[47],根据内标与待测组分[M+H]+离子的峰面积及相对校正因子来计算GDGTs含量。但实际上,C46内标与GDGTs各组分的理化性质、色谱行为、离子化效率、质谱响应等并不完全相同,因此根据其计算的GDGTs各组分含量并不十分准确。今后,随着GDGTs商用标准物质及同位素内标的出现,将改善定量分析的准确性。
不同化合物在不同仪器上或同一台仪器在不同时间检测时,会因为仪器的设置参数或状态的改变而导致响应值发生改变,因此开展GDGTs分析方法验证十分必要。2009年,Schouten等[80]组织了全球15个实验室间的比对,采用HPLC-APCI-MS法对2个海洋沉积物样品进行分析,通过比较2种GDGTs古气候指标来评估分析方法的准确性和可靠性。比对结果表明,TEX86指标在实验室内的重复性结果和实验室间的再现性结果均较好,对应的温度误差分别在1~2℃之间和3~4℃之间;而BIT指标在同一实验室内的重复性较好,但实验室间的再现性结果不理想,其原因可能是BIT指标同时涉及brGDGTs和isoGDGTs,而两者在不同质谱仪上的响应差异较大,导致BIT值有较大不同[80]。2013年,第二轮全球实验室间比对分析表明两种指标的结果均比第一次有所改善,TEX86指标的再现性温度误差降低至1.3~3.0℃,与海洋其他古温度指标的误差相近[81]。但BIT指标实验室间的再现性结果依然不理想,这可能与仪器结构、参数设置等有关。Escala等[60]和Schouten等[80-81]建议后续可采用已知BIT值的样品先对质谱进行校正,再进行BIT指标的测定。
3.2 GDGTs结构鉴定
GDGTs的结构鉴定通常借助高分辨质谱(HRMS)、气相色谱-质谱(GC-MS)和核磁共振波谱(NMR)[18,42,63,70]。依据HRMS提供的质子化分子离子峰的精确质量数及其同位素峰的相对丰度,可以推测GDGTs的分子式[77,79]。再根据质谱的一级、二级碎片离子,结合质谱裂解规律,可以推测出GDGTs可能分子结构[41-42]。但是,应用HRMS不能揭示同分异构体间的微小结构差异(如Ⅲa5与Ⅲa6)[39]。
“醚键裂解法”是将结构复杂的GDGTs转化为分子量更小、结构更简单的组分,以便进行更精准的结构解析。该法通过裂解GDGTs分子结构中的醚键,将其转化为卤代烃和甘油两部分,再将卤代烃还原为支链烷烃,之后用GC-MS对烷烃进行分析。根据特征碎片离子、保留时间、保留指数等信息,结合文献和质谱标准谱库,可以推断出烷烃部分的结构,再结合甘油的构型,最终得到GDGTs完整的分子结构[39-41,72,82-83]。在电子轰击电离源下,烷烃的支链节点通常会优先断裂,形成强度较高的特征碎片离子,这一规律可以用来判断烷烃侧链上甲基支链的位置,从而实现5-甲基、6-甲基等brGDGTs位置异构体的鉴别[39-41]。上述方法最初用于鉴定isoGDGTs的分子结构[69-70],近来也被用于brGDGTs和OH-GDGTs等物质的结构解析[39-41,82]。
常规气相色谱的工作温度上限在300~350℃之间,难以将GDGTs汽化,而高温气相色谱(HT-GC)的工作温度上限达到400~450℃,可以将GDGTs汽化而实现直接分析。Weijers等[18]和Pancost等[72]运用配有火焰离子化检测器的HT-GC,在140~420℃的程序升温下实现CL-GDGTs的分析。Sutton等[83]利用HT-GC结合飞行时间质谱仪,在20min内实现isoGDGTs的快速分析。
核磁共振氢谱(1H-NMR)、碳谱(13C-NMR)及二维核磁共振技术(如HSQC、HMBC、COSY)也是解析GDGTs结构时常用的分析手段,其提供的大量分子结构信息可与HPLC-MS、GC-MS等结果相互验证[18,44,71,82]。但NMR的局限性在于其灵敏度较低且为非选择性分析,故要求测试样品的纯度高(通常>90%),并且待测组分的含量达到mg级才能保证信号强度。但是,将GDGT单体从结构相似的同系物、异构体中分离、富集并不容易,Liu等[82]从1kg干重的海洋沉积物中仅分离出0.6mg的GDGT单体。鉴于此,目前只有个别GDGTs组分(如Ⅰa、Ⅰb、Ⅱa)的结构得到NMR验证[18,44,71]。今后,可以尝试应用天然产物、药物代谢等领域常用的HPLC-NMR联用技术,简化操作流程,实现更高效、便捷的GDGTs结构解析[84]。
3.3 GDGTs同位素分析
GDGTs的同位素组成蕴含着重要的生物和环境信息[65,85-90]。在缺氧环境中,可以通过测定沉积物中isoGDGTs的δ13C值,揭示是否存在甲烷厌氧氧化古菌[61]。湖泊沉积物和湖水悬浮颗粒物中brGDGTs的δ13C值,可以帮助判断brGDGTs的来源及其母源细菌的代谢途径[87-88]。海洋中isoGDGTs的δ13C值可以指示在古海洋生产力的演变历史[89]。海洋沉积物中isoGDGTs的14C年龄可以揭示其母源微生物的来源[68],还可以与海洋中其他生物标志物(如浮游有孔虫、长链烯酮)的年龄对比验证[90-91]。
当前,稳定碳、氢同位素分析主要采用气相色谱-同位素比值质谱(GC-C/TC-IRMS)技术[40,61,65,87-89]。在分析前,GDGTs先要经过“醚键裂解”转化为分子量更小、挥发性更强的烷烃,然后再进行GC-C/TC-IRMS分析[61,69-70,85]。Pearson等[89]利用线轴微燃烧技术建立一种直接测定isoGDGTs碳同位素的方法,避免了“醚键裂解”过程可能引起的同位素分馏效应,提高了方法的准确度和精密度(在1σ范围内误差仅为±0.25‰),但该法由于涉及仪器改装因此并不普及。Lin等[86]提出一种测定IPL-GDGTs糖苷头基δ13C的方法,该法将IPL-GDGTs水解得到的糖苷头基衍生、净化,再由GC-C-IRMS测定,结果表明糖苷头基比其烷烃骨架更富集δ13C(偏移量可达15‰),这种分子内稳定同位素分测试技术为探索GDGTs头基和骨架的代谢提供新的视角和方式。
GDGTs氢同位素组成可以指示氢的来源、重建降水、海拔等变化趋势,但目前相关研究较少[22,70]。Huguet等[22]应用稳定同位素探针技术,将富含brGDGTs的天然泥炭样品在重水和13C标记的碳酸氢钠中培养,然后利用IR-GCMS测定培养后样品brGDGTs的δD和δ13C值,首次量化了泥炭中brGDGTs的生产率。Kaneko等[70]提出了一种运用GC-IRMS测定isoGDGTs烷基侧链δD值的方法,该法通过“醚键裂解法”将isoGDGTs转化为卤代烃,利用H2/PtO2将卤代烃还原为饱和烃,再通过GC-IRMS分析获得氢同位素组成。结果表明,醚键裂解过程不存在δD分馏效应,而H2/PtO2氢化过程存在一定程度的δD分馏,但通过质量平衡校正,可以获得较准确的δD值[70]。2021年,Lengger等[92]利用HT-GC结合IRMS开发了一种可直接测定GDGTs中δD值的方法,该法不需要“醚键裂解”等过程,操作简单、分析误差小,但由于HT-GC尚未普及,因此其应用仍然有限。
近年来,放射性碳同位素技术在GDGTs高精度定年、古环境示踪、海洋碳循环等研究中逐渐兴起[68,90-91]。放射性碳同位素的分析主要借助加速器质谱仪,测试样品的最低含碳量约为100μg C[93]。为满足测试要求,一般需要从大量环境样品中提取、分离、纯化,才能获取足够量、纯度高的GDGTs单体[18,90],如Haghipour等[94]从3kg土壤样品中仅提取出15μg C。2021年,Gies等[90]提出一种无需分离GDGTs单体,仅按brGDGTs和isoGDGTs分类即可测定14C的方法,将初始的取样量降低至500g,大幅简化了前处理操作,并且对于年龄小于1800y的样品,只需20μg C的上样量就可以获得准确、精密的14C年龄结果。
4. 结语与展望
近二十年来,GDGTs的分析技术和方法取得长足进步,这为古气候重建、全球气候变化等研究提供了极大助力。目前,地质环境中GDGTs的样品前处理方法已相对成熟,以HPLC-MS、GC-MS、NMR、GC-IRMS为主的技术手段也成为分析GDGTs的重要工具,但现有方法在分离、纯化、准确定量等方面仍然存在一些问题亟待解决:①标准化问题。当前已有多种GDGTs分析方法,但尚缺乏测定GDGTs的标准化方法和程序,不同实验室和实验人员使用的前处理方法、仪器方法和数据处理方法的差异,均会影响结果的比较和重复性的验证。②方法效率问题。现有提取、分离和纯化方法较为复杂,操作难度较高;HPLC-MS定量分析用时较长,分析效率较低。③GDGTs未知组分问题。环境中仍然存在一些结构未知的GDGTs新组分。样品中这些未知组分的存在,不仅可能影响分析结果的准确性,还可能给古气候指标带来误差。
因此,建议今后分析方法的发展应重点考虑以下方面:①建立GDGTs标准分析方法。亟需开展GDGTs分析方法标准化研究,并建立相应的质量控制与评价体系,从而提高分析结果的可靠性及可比性。②开发便捷、高效的GDGTs分析方法。包括:开发分离性能更好的色谱柱,以简化现有串连2~4根色谱柱的HPLC方法;开发操作更简单、自动化程度更高的提取、分离及净化方法;运用高精度的HRMS,开发更加灵敏、准确的定性及定量方法。③开展环境中GDGTs未知组分的识别与结构解析研究。综合运用HPLC-HRMS、GC-MS、NMR等技术手段,进一步识别环境中GDGTs未知组分,从而更好地表征环境中GDGTs的分布及组成。④加强GDGTs同位素技术方法研究。亟待开发进样量更小、操作更简便的同位素技术及方法,并拓展其应用。
-
图 2 内蒙古双沟山天池湖泊沉积物中GDGTs的液相色谱总离子流图和提取离子流图
图中分析条件:在正己烷-异丙醇梯度洗脱下,以2根BEH Hilic色谱柱(150mm×2.1mm×1.7μm)和1根硅胶色谱柱(150mm×2.1mm×1.9μm)串联,经HPLC-APCI-MS分析,实现GDGTs各组分良好分离。
Figure 2. Total ion chromatogram (TIC) and extracted ion chromatogram (EIC) of GDGTs identified in lake sediment of Lake Shuanggoushan, Inner Mongolia. The chromatogram generated by HPLC-APCI-MS showing the elution order of brGDGTs and isoGDGTs with [M+H]+ ions.
Analytical condition: under gradient elution of n-hexane/isopropanol, the good separation of GDGTs were achieved by HPLC-APCI-MS method with two BEH Hilic columns (150mm×2.1mm×1.7μm) and one silica column (150mm×2.1mm×1.9μm) in tandem.
表 1 地质环境样品中GDGTs的不同提取、分离、纯化方法
Table 1 Different extraction, separation and purification methods of GDGTs in geological environment samples.
样品类型 提取方法及提取剂 分离及净化方法 参考文献 土壤 BD法,甲醇-二氯甲烷-磷酸缓冲液 硅胶柱,正己烷/乙酸乙酯、乙酸乙酯、甲醇 Pitcher等(2009) 土壤 超声萃取法,甲醇、甲醇-二氯甲烷 硅胶柱,正己烷/二氯甲烷、二氯甲烷/甲醇 Bai等(2017) 土壤、湖泊沉积物 BD法,甲醇-二氯甲烷-磷酸缓冲液 硅胶柱,正己烷/乙酸乙酯、甲醇 Buckles等(2014) 土壤、湖泊沉积物 索氏抽提法,甲醇-二氯甲烷 Al2O3柱,正己烷/二氯甲烷、二氯甲烷/甲醇 Hu等(2016) 黄土 ASE,二氯甲烷-甲醇 硅胶柱,二氯甲烷/乙酸乙酯 Lu等(2016) 泥炭 超声萃取法,二氯甲烷、二氯甲烷/甲醇 硅胶柱,正己烷/甲醇 Zheng等(2018) 泥炭 MAE,二氯甲烷-甲醇 硅胶柱,二氯甲烷/甲醇 Naafs等(2017) 海洋沉积物 索氏抽提法,甲醇-二氯甲烷 Al2O3柱,正己烷/二氯甲烷、二氯甲烷/甲醇 Liao等(2020) 海洋沉积物 BD法,甲醇-二氯甲烷-磷酸缓冲液 硅胶柱,正己烷/乙酸乙酯
制备HPLC,正己烷/异丙醇Zhu等(2013) 湖泊沉积物 ASE法,二氯甲烷-甲醇 Al2O3柱,二氯甲烷、二氯甲烷/甲醇 Chu等(2017) 湖泊沉积物 ASE法,二氯甲烷-甲醇 Al2O3柱,正己烷/二氯甲烷、二氯甲烷/甲醇 Li等(2023) 湖泊沉积物 ASE法,二氯甲烷-甲醇 Al2O3柱,二氯甲烷、二氯甲烷/甲醇
制备HPLC,正己烷/异丙醇Weber等(2015 海洋、湖泊沉积物 MAE法,二氯甲烷-甲醇 Al2O3柱,正己烷/二氯甲烷、二氯甲烷/甲醇 Escala等(2009) 湖泊水体悬浮颗粒物 BD法,甲醇-二氯甲烷-磷酸缓冲液 Al2O3柱,正己烷/二氯甲烷、二氯甲烷/甲醇 Kumar等(2019) -
[1] Castañeda I S, Schouten S. A review of molecular organic proxies for examining modern and ancient lacustrine environments[J]. Quaternary Science Reviews, 2011, 30: 2851−2891. doi: 10.1016/j.quascirev.2011.07.009
[2] 尚文郁, 孙静轶, 谢曼曼, 等. 基于Py-GC/MS的沙漠湖泊直链脂肪族化合物分析及古气候应用初探[J]. 岩矿测试, 2022, 41(5): 836−848. Shang W Y, Sun J Y, Xie M M, et al. Py-GC/MS analysis method for aliphatic biomarker in desert lake sediment and its application in paleoclimatic study[J]. Rock and Mineral Analysis, 2022, 41(5): 836−848.
[3] Schouten S, Hopmans E C, Damsté J S S. The organic geochemistry of glycerol dialkyl glycerol tetraether lipids: A review[J]. Organic Geochemistry, 2013, 54: 19−61. doi: 10.1016/j.orggeochem.2012.09.006
[4] Dong L, Li Q Y, Li L, et al. Glacial–interglacial contrast in MBT/CBT proxies in the South China Sea: Implications for marine production of branched GDGTs and continental teleconnection[J]. Organic Geochemistry, 2015, 79: 74−82. doi: 10.1016/j.orggeochem.2014.12.008
[5] Ge H M, Zhang C L. Advances in GDGT research in Chinese marginal seas: A review[J]. Science China: Earth Sciences, 2016, 59(6): 1173−1186. doi: 10.1007/s11430-015-5242-z
[6] de Jonge C, Stadnitskaia A, Cherkashov G, et al. Branched glycerol dialkyl glycerol tetraethers and crenarchaeol record post-glacial sea level rise and shift in source of terrigenous brGDGTs in the Kara Sea (Arctic Ocean)[J]. Organic Geochemistry, 2016, 92: 42−54. doi: 10.1016/j.orggeochem.2015.11.009
[7] Cao J T, Rao Z G, Shi F X, et al. [J]. Ice formation on lake surfaces in winter causes warm-season bias of lacustrine brGDGT temperature estimates[J]. Biogeosciences, 2020, 17: 2521–2536.
[8] Wang H Y, Liu W G, He Y X, et al. Salinity-controlled isomerization of lacustrine brGDGTs impacts the associated MBT’5ME terrestrial temperature index[J]. Geochimica et Cosmochimica Acta, 2021, 305: 33−48. doi: 10.1016/j.gca.2021.05.004
[9] Feng X P, Zhao C, D’Anderea W J, et al. Temperature fluctuations during the Common Era in subtropical Southwestern China inferred from brGDGTs in a remote alpine lake[J]. Earth and Planetary Science Letters, 2019, 510: 26−36. doi: 10.1016/j.jpgl.2018.12.028
[10] 李婧婧, 杨欢, 郑峰峰, 等. 湖泊水体微生物四醚膜脂化合物研究进展[J]. 湖泊科学, 2021, 33(5): 1334−1349. doi: 10.18307/2021.0504 Li J J, Yang H, Zheng F F, et al. Occurrence and distribution of glycerol dialkyl glycerol tetraethers in lake water column: A review[J]. Journal of Lake Sciences, 2021, 33(5): 1334−1349. doi: 10.18307/2021.0504
[11] Cheng Z Y, Yu F L, Ruan X Y, et al. GDGTs as indicators for organic-matter sources in a small subtropical river-estuary system[J]. Organic Geochemistry, 2021, 153: 104180. doi: 10.1016/j.orggeochem.2021.104180
[12] Pitcher A, Schouten S, Damste J S S. In situ production of crenarchaeol in two California hot springs[J]. Applied and Environmental Microbiology, 2009, 75: 4443−4451. doi: 10.1128/AEM.02591-08
[13] Wu W Y, Zhang C L, Wang H Y, et al. Impacts of temperature and pH on the distribution of archaeal lipids in Yunnan hot springs, China[J]. Frontiers in Microbiology, 2013, 4: 312.
[14] Ding S, Lange M, Lipp J, et al. Characteristics and origin of intact polar lipids in soil organic matter[J]. Soil Biology and Biochemistry, 2020, 151: 108045. doi: 10.1016/j.soilbio.2020.108045
[15] de Jonge C, Radujkovic D, Sigurdsson B D, et al. Lipid biomarker temperature proxy responds to abrupt shift in the bacterial community composition in geothermally heated soils[J]. Organic Geochemistry, 2019, 137: 103897. doi: 10.1016/j.orggeochem.2019.07.006
[16] Cao M, Rueda G, Rivas-Ruiz P, et al. Branched GDGT variability in sediments and soils from catchments with marked temperature seasonality[J]. Organic Geochemistry, 2018, 122: 98−114. doi: 10.1016/j.orggeochem.2018.05.007
[17] Peaple M D, Beverly E J, Garza B, et al. Identifying the drivers of GDGT distributions in alkaline soil profiles within the Serengeti ecosystem[J]. Organic Geochemistry, 2022, 169: 104433. doi: 10.1016/j.orggeochem.2022.104433
[18] Weijers J W H, Schouten S, Hopmans E C, et al. Membrane lipids of mesophilic anaerobic bacteria thriving in peats have typical archaeal traits[J]. Environmental Microbiology, 2006, 8(4): 648−657. doi: 10.1111/j.1462-2920.2005.00941.x
[19] 樊嘉琛, 钱施, 裴宏业, 等. 微生物醚类化合物在泥炭古环境重建中的应用: 进展与问题[J]. 地球科学进展, 2021, 36(12): 1272−1290. doi: 10.11867/j.issn.1001-8166.2021.095 Fan J C, Qian S, Pei H Y, et al. Application of microbial ether lipids in the reconstruction of paleoenvironments in peatlands: Progress and problems[J]. Advances in Earth Science, 2021, 36(12): 1272−1290. doi: 10.11867/j.issn.1001-8166.2021.095
[20] 李奇缘, 刘潇敏, 王章章, 等. 青藏高原东部现代泥炭GDGTs分布特征及环境意义[J]. 第四纪研究, 2016, 36(2): 388−395. Li Q Y, Liu X M, Wang Z Z, et al. Distributions and environmental significance of GDGTs in modern peat samples from Eastern Tibetan Plateau[J]. Quaternary Sciences, 2016, 36(2): 388−395.
[21] Naafs B D A, Inglis G N, Zhang Y, et al. Introducing global peat-specific temperature and pH calibrations based on brGDGT bacterial lipids[J]. Geochimica et Cosmochimica Acta, 2017, 208: 285−301. doi: 10.1016/j.gca.2017.01.038
[22] Huguet A, Meador T B, Laggoun-Défarge F, et al. Production rates of bacterial tetraether lipids and fatty acids in peatland under varying oxygen concentrations[J]. Geochimica et Cosmochimica Acta, 2017, 203: 103−116. doi: 10.1016/j.gca.2017.01.012
[23] 梁栋, 李丽, 贺娟, 等. 运用生物标志物重建古盐度的研究进展[J]. 地球化学, 2022, 51(3): 316−332. Liang D, Li L, He J, et al. Progress in paleosalinity reconstruction: A review of biomarker approaches[J]. Geochimica, 2022, 51(3): 316−332.
[24] Ernst R, Ejsing C S, Antonny B. Homeoviscous adaptation and the regulation of membrane lipids[J]. Journal of Molecular Biology, 2016, 428(24): 4776−4791. doi: 10.1016/j.jmb.2016.08.013
[25] Summons R E, Welander P V, Gold D A. Lipid biomarkers: Molecular tools for illuminating the history of microbial life[J]. Nature Reviews Microbiology, 2022, 20: 174−185. doi: 10.1038/s41579-021-00636-2
[26] Halamka T A, Raberg J H, McFarlin J M, et al. Production of diverse brGDGTs by acidobacterium Solibacter usitatus in response to temperature, pH, and O2 provides a culturing perspective on brGDGT proxies and biosynthesis[J]. Geobiology, 2022, 21: 102−118.
[27] Chen Y F, Zheng F F, Yang H, et al. The production of diverse brGDGTs by an acidobacterium providing a physiological basis for paleoclimate proxies[J]. Geochimica et Cosmochimica Acta, 2022, 337(15): 155−165.
[28] Naafs B D A, Oliveira A S F, Mulholland A J. Molecular dynamics simulations support the hypothesis that the brGDGT paleothermometer is based on homeoviscous adaptation[J]. Geochimica et Cosmochimica Acta, 2021, 312: 44−56. doi: 10.1016/j.gca.2021.07.034
[29] Jenkyns H C, Schouten-Huibers L, Schouten S, et al. Warm middle Jurassic-early Cretaceous high-latitude sea-surface temperatures from the Southern Ocean[J]. Climate of the Past, 2012, 8(1): 215−226. doi: 10.5194/cp-8-215-2012
[30] Kielhofer J R, Tierney J E, Reuther J D, et al. BrGDGT temperature reconstruction from interior Alaska: Assessing 14, 000 years of deglacial to Holocene temperature variability and potential effects on early human settlement[J]. Quaternary Science Reviews, 2023, 303: 107979. doi: 10.1016/j.quascirev.2023.107979
[31] Chu G Q, Sun Q, Zhu Q Z, et al. The role of the Asian winter monsoon in the rapid propagation of abrupt climate changes during the last deglaciation[J]. Quaternary Science Reviews, 2017, 177: 120−129. doi: 10.1016/j.quascirev.2017.10.014
[32] Bai Y, Chen C H, Xu Q, et al. Paleoaltimetry potentiality of branched GDGTs from Southern Tibet[J]. Geochemistry, Geophysics, Geosystems, 2018, 19: 551−564. doi: 10.1002/2017GC007122
[33] Liu X L, Summons R E, Hinrichs K U. Extending the known range of glycerol ether lipids in the environment: Structural assignments based on tandem mass spectral fragmentation patterns[J]. Rapid Communications in Mass Spectrometry, 2012, 26(19): 2295−2302. doi: 10.1002/rcm.6355
[34] Li Y J, Su X, Jiao L, et al. Intact polar and core tetraether lipids in sediments from the haiyang 4 cold-seep of the Northern South China Sea and their implications[J]. Acta Geologica Sinica (English Edition), 2022, 96(2): 691−700. doi: 10.1111/1755-6724.14928
[35] Kumar D M, Woltering M, Hpmans E C, et al. The vertical distribution of Thaumarchaeota in the water column of Lake Malawi inferred from core and intact polar tetraether lipids[J]. Organic Geochemistry, 2019, 132: 37−49. doi: 10.1016/j.orggeochem.2019.03.004
[36] Villanueva L, Sinninghe Damsté J S, Schouten S. A re-evaluation of the archaeal membrane lipid biosynthetic pathway[J]. Nature Reviews Microbiology, 2014, 12(6): 438−448. doi: 10.1038/nrmicro3260
[37] Zeng Z R, Liu X L, Farley K R, et al. GDGT cyclization proteins identify the dominant archaeal sources of tetraether lipids in the ocean[J]. Proceedings of the National Academy of Sciences, 2019, 116(45): 22505−22511.
[38] Yang W, Chen H H, Chen Y F, et al. Thermophilic archaeon orchestrates temporal expression of GDGT ring synthases in response to temperature and acidity stress[J]. Environmental Microbiology, 2023, 25(2): 575−587. doi: 10.1111/1462-2920.16301
[39] De Jonge C, Hopmans E C, Stadnitskaia A, et al. Identification of novel penta- and hexamethylated branched glycerol dialkyl glycerol tetraethers in peat using HPLC-MS2, GC-MS and GC–SMB-MS[J]. Organic Geochemistry, 2013, 54: 78−82. doi: 10.1016/j.orggeochem.2012.10.004
[40] Weber Y, De Jonge C, Rijpstra W I C, et al. Identification and carbon isotope composition of a novel branched GDGT isomer in lake sediments: Evidence for lacustrine branched GDGT production[J]. Geochimica et Cosmochimica Acta, 2015, 154: 118−129. doi: 10.1016/j.gca.2015.01.032
[41] Ding S, Schwab V F, Ueberschaar N, et al. Identification of novel 7-methyl and cyclopentenyl branched glycerol dialkyl glycerol tetraethers in lake sediments[J]. Organic Geochemistry, 2016, 102: 52−58. doi: 10.1016/j.orggeochem.2016.09.009
[42] Liu X L, Russell D A, Bonfio C, et al. Glycerol configurations of environmental GDGTs investigated using a selective sn2 ether cleavage protocol[J]. Organic Geochemistry, 2019, 128: 57−62. doi: 10.1016/j.orggeochem.2018.12.003
[43] Sinninghe Damsté J S, Rijpstra W I C, Hopmans E C, et al. The enigmatic structure of the crenarchaeol isomer[J]. Organic Geochemistry, 2018, 124: 22−28. doi: 10.1016/j.orggeochem.2018.06.005
[44] Sinninghe Damsté J S, Hopmans E C, Pancost R D, et al. Newly discovered non–isoprenoid glycerol dialkyl glycerol tetraether lipids in sediments[J]. Chemical Communications, 2000, 17: 1683−1684.
[45] Bale N J, Palatinszky M, Rijpstra W I C, et al. Membrane lipid composition of the moderately thermophilic ammonia-oxidizing archaeon “Candidatus Nitrosotenuis uzonensis” at different growth temperatures[J]. Applied and Environmental Microbiology, 2019, 85(20): e01332−19.
[46] Sinninghe Damsté J S, Rijpstra W I C, Hopmans E C, et al. Ether- and ester-bound iso-diabolic acid and other lipids in members of acidobacteria subdivision 4[J]. Applied and Environmental Microbiology, 2014, 80: 5207−5218. doi: 10.1128/AEM.01066-14
[47] Halamka T A, McFarlin J M, Younkin A D, et al. Oxygen limitation can trigger the production of branched GDGTs in culture[J]. Geochemical Perspectives Letters, 2021, 19: 36−39.
[48] Liang J, Richter N, Xie H C, et al. Branched glycerol dialkyl glycerol tetraether (brGDGT) distributions influenced by bacterial community composition in various vegetation soils on the Tibetan Plateau[J]. Palaeogeography, Palaeoclimatology, Palaeoecology, 2023, 611: 111358. doi: 10.1016/j.palaeo.2022.111358
[49] Buckles L K, Weijers J W H, Verschuren D, et al. Sources of core and intact branched tetraether membrane lipids in the lacustrine environment: Anatomy of Lake Challa and its catchment, equatorial East Africa[J]. Geochimica et Cosmochimica Acta, 2014, 140: 106−126. doi: 10.1016/j.gca.2014.04.042
[50] 王欢业, 刘卫国, 张传伦. 超声波有机溶剂萃取法和改进的Bligh-Dyer 法提取甘油二烷基甘油四醚类化合物效果对比[J]. 地球环境学报, 2017, 8(2): 176−184. Wang H Y, Liu W G, Zhang C L. Comparison of the ultrasound-assisted organic solvent extraction and modified Bligh-Dyer extraction for the analysis of glycerol dialkyl glycerol tetraethers from environmental samples[J]. Journal of Earth Environment, 2017, 8(2): 176−184.
[51] Schouten S, Huguet C, Hopmans E C, et al. Analytical methodology for TEX86 paleothermometry by high-performance liquid chromatography/atmospheric pressure chemical ionization-mass spectrometry[J]. Analytical Chemistry, 2007, 79(7): 2940−2944. doi: 10.1021/ac062339v
[52] Zheng Y H, Pancost R D, Naafs B D A, et al. Transition from a warm and dry to a cold and wet climate in NE China across the Holocene[J]. Earth and Planetary Science Letters, 2018, 493: 36−46. doi: 10.1016/j.jpgl.2018.04.019
[53] Hu J F, Zhou H D, Peng P A, et al. Seasonal variability in concentrations and fluxes of glycerol dialkyl glycerol tetraethers in Huguangyan Maar Lake, SE China: Implications for the applicability of the MBT–CBT paleotemperature proxy in lacustrine settings[J]. Chemical Geology, 2016, 420: 200−212. doi: 10.1016/j.chemgeo.2015.11.008
[54] Liao W S, Hu J F, Zhou H D, et al. Climatic and human impact on the environment: Insight from the tetraether lipid temperature reconstruction in the Beibu Gulf, China[J]. Quaternary International, 2020, 536: 75−84. doi: 10.1016/j.quaint.2019.12.004
[55] 童晓宁. 松辽盆地及邻区晚白垩世四醚类脂物GDGTs揭示的古气候/环境意义[D]. 广州: 中国科学院广州地球化学研究所, 2018: 44-46. Tong X N. The paleoclimate and paleoenvironment reconstruction based on glycerol dialkyl glycerol tetraethers during late Cretaceous in the Songliao Basin and its vicinity[D]. Guangzhou: Guangzhou Institute of Geochemistry, Chinese Academy of Sciences, 2018: 44-46.
[56] Lengger S K, Sutton P A, Rowland S J, et al. Archaeal and bacterial glycerol dialkyl glycerol tetraether (GDGT) lipids in environmental samples by high temperature-gas chromatography with flame ionisation and time-of-flight mass spectrometry detection[J]. Organic Geochemistry, 2018, 121: 10−21. doi: 10.1016/j.orggeochem.2018.03.012
[57] Li Q, Sun Q, Xie M M, et al. Coupled temperature variations in the Huguangyan Maar Lake between high and low latitude[J]. Quaternary Science Reviews, 2023, 305: 108011. doi: 10.1016/j.quascirev.2023.108011
[58] Lu H X, Liu W G, Wang H Y, et al. Variation in 6-methyl branched glycerol dialkyl glycerol tetraethers in Lantian loess–paleosol sequence and effect on paleotemperature reconstruction[J]. Organic Geochemistry, 2016, 100: 10−17. doi: 10.1016/j.orggeochem.2016.07.006
[59] Huguet C, Martens-Habbena M, Urakawa H, et al. Comparison of extraction methods for quantitative analysis of core and intact polar glycerol dialkyl glycerol tetraethers (GDGTs) in environmental samples[J]. Limnology and Oceanography:Methods, 2010, 8: 127−145. doi: 10.4319/lom.2010.8.127
[60] Escala M, Fietz S, Rueda G, et al. Analytical Considerations for the use of the paleothermometer tetraether index86 and the branched vs isoprenoid tetraether index regarding the choice of cleanup and instrumental conditions[J]. Analytical Chemistry, 2009, 81: 2701−2707. doi: 10.1021/ac8027678
[61] Oba M, Sakata S, Tsunogai U. Polar and neutral isopranyl glycerol ether lipids as biomarkers of archaea in near-surface sediments from the Nankai Trough[J]. Organic Geochemistry, 2006, 37: 1643−1654. doi: 10.1016/j.orggeochem.2006.09.002
[62] Auderset A, Schmitt M, Martínez-García A. Simultaneous extraction and chromatographic separation of n-alkanes and alkenones from glycerol dialkyl glycerol tetraethers via selective accelerated solvent extraction[J]. Organic Geochemistry, 2020, 143: 103979. doi: 10.1016/j.orggeochem.2020.103979
[63] Lengger S K, Hopmans E C, Sinninghe Damsté J S, et al. Comparison of extraction and work up techniques for analysis of core and intact polar tetraether lipids from sedimentary environments[J]. Organic Geochemistry, 2012, 47: 34−40. doi: 10.1016/j.orggeochem.2012.02.009
[64] Yang Y, Gao C, Dang X Y, et al. Assessing hydroxylated isoprenoid GDGTs as a paleothermometer for the tropical South China Sea[J]. Organic Geochemistry, 2018, 115: 156−165. doi: 10.1016/j.orggeochem.2017.10.014
[65] Weijers J W H, Wiesenberg G L B, Hopmans E C, et al. Carbon isotopic composition of branched tetraether membrane lipids in soils suggest a rapid turnover and a heterotrophic life style of their source organism(s)[J]. Biogeosciences, 2010, 7: 2959−2973. doi: 10.5194/bg-7-2959-2010
[66] Zhu C, Lipp J S, Wörmer L, et al. Comprehensive glycerol ether lipid fingerprints through a novel reversed phase liquid chromatography–mass spectrometry protocol[J]. Organic Geochemistry, 2013, 65: 53−62. doi: 10.1016/j.orggeochem.2013.09.012
[67] Sanchi L, Ménot G, Bard E. An automated purification method for archaeal and bacterial tetraethers in soils and sediments[J]. Organic Geochemistry, 2013, 54: 83−90. doi: 10.1016/j.orggeochem.2012.10.005
[68] Shah S R, Mollenhauer G, Ohkouchi N, et al. Origins of archaeal tetraether lipids in sediments: Insights from radiocarbon analysis[J]. Geochimica et Cosmochimica Acta, 2008, 72: 4577−4594. doi: 10.1016/j.gca.2008.06.021
[69] Schouten S, Hoefs M J L, Koopmans M P, et al. Structural characterization, occurrence and fate of archaeal ether-bound acyclic and cyclic biphytanes and corresponding diols in sediments. Organic Geochemistry, 1998, 29: 5-7, 1305-1319.
[70] Kaneko M, Kitajima F, Naraoka H. Stable hydrogen isotope measurement of archaeal ether-bound hydrocarbons[J]. Organic Geochemistry, 2011, 42: 166−172. doi: 10.1016/j.orggeochem.2010.11.002
[71] Sinninghe Damsté J S, Schouten S, Ellen Hopmans et al. Crenarchaeol: The characteristic core glycerol dibiphytanyl glycerol tetraether membrane lipid of cosmopolitan pelagic crenarchaeota[J]. Journal of Lipid Research, 2002, 43(10): 1641−1651. doi: 10.1194/jlr.M200148-JLR200
[72] Pancost R D, Coleman J M, Love C D, et al. Kerogen-bound glycerol dialkyl tetraether lipids released by hydropyrolysis of marine sediments: A bias against incorporation of sedimentary organisms?[J]. Organic Geochemistry, 2008, 39(9): 1359−1371. doi: 10.1016/j.orggeochem.2008.05.002
[73] Hopmans E C, Schouten S, Sinninghe Damsté J S, et al. The effect of improved chromatography on GDGT-based palaeoproxies[J]. Organic Geochemistry, 2016, 93: 1−6. doi: 10.1016/j.orggeochem.2015.12.006
[74] Yang H, Lü X X, Ding W H, et al. The 6-methyl branched tetraethers significantly affect the performance of the methylation index (MBT’) in soils from an altitudinal transect at Mount Shennongjia[J]. Organic Geochemistry, 2015, 82: 42−53. doi: 10.1016/j.orggeochem.2015.02.003
[75] Chen Y, Zhang C, Jia C, et al. Tracking the signals of living archaea: A multiple reaction monitoring (MRM) method for detection of trace amounts of intact polar lipids from the natural environment[J]. Organic Geochemistry, 2016, 97: 1−4. doi: 10.1016/j.orggeochem.2016.04.006
[76] Davtian N, Bard E, Ménot G, et al. The importance of mass accuracy in selected ion monitoring analysis of branched and isoprenoid tetraethers[J]. Organic Geochemistry, 2018, 118: 58−62. doi: 10.1016/j.orggeochem.2018.01.007
[77] 李运运, 何晨, 吴建勋, 等. 甘油四醚类化合物的高分辨质谱表征[J]. 质谱学报, 2021, 42(6): 1127−1138. Li Y Y, He C, Wu J X, et al. Molecular characterization of glycerol dialkyl glycerol tetraethers by high resolution orbitrap mass spectrometry[J]. Journal of Chinese Mass Spectrometry Society, 2021, 42(6): 1127−1138.
[78] Horai S, Yamauchi N, Naraoka H. Simultaneous total analysis of core and polar membrane lipidsin archaea by high-performance liquid chromatography/high-resolution mass spectrometry coupled with heated electrospray ionization[J]. Rapid Communications in Mass Spectrometry, 2019, 33(20): 1571−1577. doi: 10.1002/rcm.8506
[79] Radović J R, Silva R C, Snowdon R. Rapid screening of glycerol ether lipid biomarkers in recent marine sediment using atmospheric pressure photoionization in positive mode fourier transform ion cyclotron resonance mass spectrometry[J]. Analytical Chemistry, 2016, 88: 1128−1137. doi: 10.1021/acs.analchem.5b02571
[80] Schouten S, Hopmans E C, van der Meer J, et al. An interlaboratory study of TEX86 and BIT analysis using high-performance liquid chromatography-mass spectrometry[J]. Geochemistry, Geophysics, Geosystems, 2009, 10: Q03012.
[81] Schouten S, Hopmans E C, Rosell M A, et al. An interlaboratory study of TEX86 and BIT analysis of sediments, extracts, and standard mixtures[J]. Geochemistry Geophysics Geosystems, 2013, 14(12): 5263−5285. doi: 10.1002/2013GC004904
[82] Liu X L, Lipp J S, Simpson J H, et al. Mono- and dihydroxyl glycerol dibiphytanyl glycerol tetraethers in marine sediments: Identification of both core and intact polar lipid forms[J]. Geochimica et Cosmochimica Acta, 2012, 89: 102−115. doi: 10.1016/j.gca.2012.04.053
[83] Sutton P A, Rowland S J. High temperature gas chromatography-time-of-flight-mass spectrometry (HTGC-ToF-MS) for high-boiling compounds[J]. Journal of Chromatography A, 2012, 1243: 69−80. doi: 10.1016/j.chroma.2012.04.044
[84] 张何, 黄桂兰, 袁铃, 等. 液相色谱-核磁共振联用技术研究进展[J]. 化学分析计量, 2017, 26(3): 117−122. Zhang H, Huang G L, Yuan L, et al. Advances of liquid chromatography coupled with nuclear magnetic resonance spectrometer[J]. Chemical Analysis and Meterage, 2017, 26(3): 117−122.
[85] 黄咸雨, 张一鸣. 脂类单体碳同位素在湖沼古环境和古生态重建中的研究进展[J]. 地球科学进展, 2019, 34(1): 20−33. Huang X Y, Zhang Y M. An overview of the applications of lipid carbon isotope compositions in the paleoenvironmental and paleoecological reconstructions in lacustrine and peat deposits[J]. Advances in Earth Science, 2019, 34(1): 20−33.
[86] Lin Y S, Lipp J S, Yoshinaga M Y, et al. Intramolecular stable carbon isotopic analysis of archaeal glycosyl tetraether lipids[J]. Rapid Communications in Mass Spectrometry, 2010, 24: 2817−2826. doi: 10.1002/rcm.4707
[87] Weber Y, Sinninghe Damsté J S, Zopfi J, et al. Redox-dependent niche differentiation provides evidence for multiple bacterial sources of glycerol tetraether lipids in lakes[J]. Proceedings of the National Academy of Sciences of the United States of America, 2018, 115,43: 10926−10931.
[88] Colcord D E, Pearson A, Brassell S C. Carbon isotopic composition of intact branched GDGT core lipids in Greenland lake sediments and soils[J]. Organic Geochemistry, 2017, 110: 25−32. doi: 10.1016/j.orggeochem.2017.04.008
[89] Pearson A, Hurley S J, Walter S R S, et al. Stable carbon isotope ratios of intact GDGTs indicate heterogeneous sources to marine sediments[J]. Geochimica et Cosmochimica Acta, 2016, 181: 18−35. doi: 10.1016/j.gca.2016.02.034
[90] Gies H, Hagedorn F, Lupker M, et al. Millennial-age glycerol dialkyl glycerol tetraethers (GDGTs) in forested mineral soils: 14C-based evidence for stabilization of microbial necromass[J]. Biogeosciences, 2021, 18: 189−205. doi: 10.5194/bg-18-189-2021
[91] Kusch S, Rethemeyer J, Hopmans E C, et al. Factors influencing 14C concentrations of algal and archaeal lipids and their associated sea surface temperature proxies in the Black Sea[J]. Geochimica et Cosmochimica Acta, 2016, 188: 35−57. doi: 10.1016/j.gca.2016.05.025
[92] Lengger S K, Weber Y, Taylor K W R, et al. Determination of the δ2H values of high molecular weight lipids by high-temperature gas chromatography coupled to isotope ratio mass spectrometry[J]. Rapid Communications in Mass Spectrometry, 2021, 35: e8983. doi: 10.1002/rcm.8983
[93] 张海龙, 陶舒琴, 于蒙, 等. 生物标志物单体放射性碳同位素分析技术的发展[J]. 地球科学进展, 2017, 32(11): 79−89. Zhang H L, Tao S Q, Yu M, et al. A review on techniques and applications of biomarker compound-specific radiocarbon analysis[J]. Advances in Earth Science, 2017, 32(11): 79−89.
[94] Haghipour N, Ausin B, Usman M O, et al. Compound-specific radiocarbon analysis (CSRA) by elemental analyzer-accelerator mass spectrometry (EA-AMS): Precision and limitations[J]. Analytical Chemistry, 2019, 91,3: 2042−2049.
-
期刊类型引用(2)
1. 祁晓鹏,周伟,徐磊,张嘉升,杨杰. 扬子板块北缘镇巴地区下二叠统梁山组富锂黏土岩地质特征及成因初探. 矿产勘查. 2025(01): 129-141 .  百度学术
百度学术
2. 刘忠梅,周安梁. X射线衍射法快速测定铜精矿矿物组分的试验研究. 中国资源综合利用. 2024(05): 28-32 .  百度学术
百度学术
其他类型引用(1)
-
其他相关附件




 下载:
下载:
 京公网安备 11010202008159号
京公网安备 11010202008159号