Application of Specific Surface Area Nitrogen Adsorption Method to Characterize the Alkaline Dissolution of Montmorillonite
-
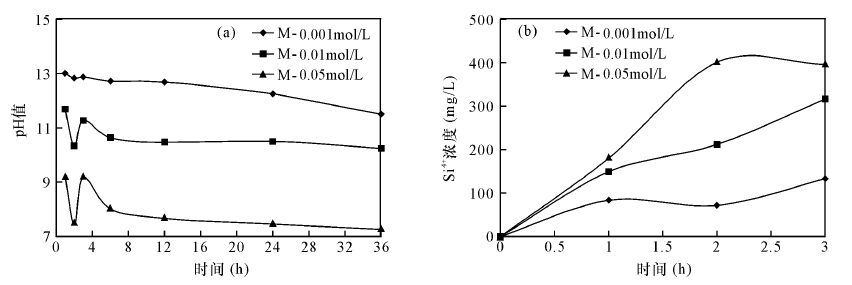
摘要: 蒙脱石矿物颗粒粒径小、比表面积大、水敏性强,易堵塞储层孔隙和喉道,是影响油田储层质量的主要黏土矿物,碱性条件下蒙脱石的溶蚀特征是油田开发中普遍关注的问题。对于蒙脱石溶蚀特征的表征,前人常用的方法有反应前后质量对比法、X射线衍射法、溶液离子浓度分析法及pH值分析法,质量对比法操作复杂,X射线衍射图谱计算人为误差大,溶液离子浓度法对难熔元素的检测误差大,pH值法受温度变化的影响。本文应用比表面积氮气吸附法测量蒙脱石在不同氢氧化钠浓度条件下的比表面积、孔容、孔径变化及吸附脱附曲线来表征其碱性溶蚀特征,优选出适合油田开发的氢氧化钠溶液浓度及充注时间(反应时间)等实验条件,确定了最佳溶蚀浓度为0.05 mol/L氢氧化钠溶液,最佳反应时间为3 h。实验表明,比表面积氮气吸附法测得的比表面积、孔容、孔径及吸附脱附曲线表征的蒙脱石溶蚀特征,与反应后溶液pH值及离子(如Si4+)浓度变化所得的结论相同,验证了该方法的正确性,且具有直观、准确、可靠的特点,可应用于比表面积较大的多孔矿物溶蚀研究。Abstract: Montmorillonite is characterized by small particle size, large specific surface area and strong water sensitivity, which results in blocking the pore and throat of the reservoir and in turn affecting its quality. Dissolution characteristics of montmorillonite under alkaline conditions are common concerns in the development of oil fields. Mass contrast, X-ray Diffraction, solution concentration analysis and value analysis of pH methods are commonly used to characterize dissolution of montmorillonite. However, the quality comparison method is complex. Calculation of diffraction pattern by the X-ray Diffraction involves large manual operation errors. The ion concentration analysis method shows a large detection error for infusible elements. Value analysis of the pH method is influenced by temperature changes. The specific surface area, pore volume, pore diameter change, adsorption-desorption curve measured by nitrogen adsorption method was used to characterize the dissolution of montmorillonite under alkaline conditions and is reported in this paper. The experimental conditions were optimized, including the concentration of NaOH solution and filling time (reaction time). The best concentration of NaOH solution was 0.05 mol/L and the best reaction time was 3 h. Results show that the dissolved results of montmorillonite by the nitrogen adsorption method characterized by specific surface area, pore volume, pore diameter and adsorption desorption curve are consistent with the results by the change of the pH value and the concentration of the solution (such as Si4+). Therefore, the method is feasible and can be used to characterize the dissolution of minerals with multiple pores and large specific surface area.
-
在宝石学中,主要由低温石英(α-石英)微晶组成且具有一定工艺价值的矿物集合体通常被称为石英质玉石。该类玉石分布十分广泛,并凭借其多彩的颜色、温润细腻的质地而深受人们青睐,具有较高的市场潜力,从而推动了石英质玉石的相关研究。石英质玉石的成分往往对其外观特征、品质级别、应用性能等方面有着显著影响,因此该领域也是石英质玉石研究中的热点。随着研究的不断深入,人们发现石英质玉石中还可存在一种低温低压SiO2矿物——斜硅石[1]。1976年斜硅石首次发现于西班牙大加纳利岛(Gran Canaria)Mogan组底层流纹质凝灰岩的裂隙和孔洞中[2],其晶体结构可以看作通过左形α-石英与右形α-石英的(1011) 面网交替堆叠,形成一个由SiO4四面体共角连接而成的三维结构,在单个晶胞范围内这种结构类似于作周期性重复的巴西双晶[3]。2007年国际矿物学会新矿物和矿物命名委员会正式批准,将斜硅石作为一种具有与石英相似的AB2结构的新矿物种(CNMMN No.99-035) 列入矿物名录。斜硅石和α-石英可共存于常温常压环境中,但两者具有不同的晶体结构:斜硅石的晶胞参数a=0.8758 nm,b=0.4876 nm,c=1.0715 nm,β=90.08°,其空间群为I2/a,属单斜晶系(由于β=90.08°,外观常呈假斜方对称),其对称性低于α-石英(α-石英的晶胞参数a=0.4913 nm,b=0.4913 nm,c=0.5404 nm,空间群为P3121,属三方晶系)[2, 3]。
斜硅石常以纳米尺度微晶的形式存在于各种产状、各种颜色以及不同结晶程度的石英质玉石中[1, 3, 4, 5],仅凭肉眼观察一般无法将其鉴别出来。迄今为止,斜硅石的研究主要涉及晶体结构[2, 3, 6, 7]、形态特征[1, 4, 5]、物理化学性质[1, 3]、检测方法[8, 9, 10, 11, 12, 13, 14, 15]、分布特征[1, 7, 16]和形成机理[17, 18, 19, 20, 21, 22]等方面,但关于斜硅石的相对含量对石英质玉石结晶程度的影响还未见报道,开展这些方面的研究将为大致判断石英质玉石的结晶程度提供新思路,也可为石英质玉石的品质评价、综合利用等建立科学依据和理论约束。
本文选取中国云南龙陵、安徽大别山地区、广西贺州、内蒙古阿拉善盟地区4个代表性产地所产出的不同颜色、不同结构类型的石英质玉石为研究对象,分别利用拉曼光谱、红外光谱、X射线粉晶衍射三种宝玉石研究中较为常用的测试方法,探讨了石英质玉石中斜硅石和α-石英的相对含量与石英质玉石结晶程度之间的关系,并结合斜硅石与α-石英的晶体结构特征给予了理论上的解释。
1. 实验部分
1.1 实验样品
本次研究的19件样品是从100件来自不同产地(云南龙陵、安徽大别山地区、广西贺州和内蒙古阿拉善盟地区)的石英质玉石中挑选出来的典型样品,通过偏光显微镜对样品磨制的岩石薄片进行分析,研究样品的显微结构可分为粒状结构和纤维状结构两大类。
1.2 样品分析方法及其测量条件
(1) 拉曼光谱分析:采用Renishaw-inVia型激光共聚焦显微拉曼光谱仪(英国雷尼绍公司)。仪器测量条件为:激发光源波长532 nm,分辨率1 cm-1,激光功率10 mW,测量范围100~1200 cm-1,扫描次数为3次,对测试图谱进行峰形拟合所使用的软件为Wire 4.1。
(2) 红外漫反射光谱分析:采用NICOLET iS5傅里叶变换红外光谱仪(美国尼高力公司)的PIKE TECHNOLOGIES UplRTM红外漫反射附件对19件样品进行测试,所有测试图谱均进行K-K变换予以校正。仪器主要测量参数为:扫描范围400~2000 cm-1,扫描次数为32次,分辨率4 cm-1。
(3) X射线衍射分析:采用Bruker D8 Advance型6.5kW转靶X射线衍射仪(美国Bruker公司)进行测试。根据研究内容的不同,测量参数设置略有差异:在物相鉴定时测量角度范围为10°~80°,扫描速度4°/min,步长0.02°;而在结晶度测量时角度范围为67°~69°,扫描速度0.25°/min。为尽可能地减少晶体粒度和择优取向对测量结果的影响,将样品均磨制成粒度为74 μm(200目)以下的粉末。
2. 结果与讨论
2.1 斜硅石及其相对含量表征
2.1.1 拉曼光谱分析
前人研究表明,斜硅石具有19个独立的拉曼光谱谱峰,其中多数与α-石英重合,最特征的也是最强的斜硅石拉曼特征峰位于502 cm-1附近,该峰主要与硅氧四面体组成的四方环中Si—O—Si的对称伸缩-弯曲振动有关,因此常用于确定是否有斜硅石存在[9, 12, 23]。由于石英质玉石中常含有黄色、红色等主要由铁的氧化物(氢氧化物)组成的致色物质,这些杂质矿物会对斜硅石的拉曼光谱产生影响,如赤铁矿在496 cm-1附近具有特征峰。因此,本次研究选取无色或接近无色的样品进行拉曼光谱测试,结果显示不同样品所包含的502 cm-1谱峰的强度差异较大,说明不同样品中斜硅石的相对含量存在差异。样品中的斜硅石含量越高,其拉曼光谱中斜硅石502 cm-1特征峰就越为明显。
斜硅石含量的多少可以根据研究样品的拉曼光谱和X射线粉晶衍射图谱的分析和计算获得。GÖtze等(1998)[9]配制了不同含量比例的斜硅石/α-石英样品,对其进行拉曼光谱测试,发现斜硅石在样品中的相对含量越高,拉曼光谱中502 cm-1/465 cm-1谱峰面积的比值和峰强度的比值也越高。本文利用高斯函数及洛伦兹函数对502 cm-1和465 cm-1进行峰形拟合,分别求出两峰的积分面积比(A502/A465,%)和强度比(I502/I465,%), 见表 1。有必要说明的是,由于部分样品的502 cm-1特征峰较小,无法进行较好的峰形拟合,从而计算得出的积分面积比的误差也相对较大,因此表 1中仅列出了这些样品的两峰强度比I502/I465。
表 1 不同产地石英质玉石样品的拉曼光谱和红外漫反射光谱信息Table 1. Data of Raman spectra and infrared diffuse reflectance spectra of quartzite jade samples from different geographic origins样品产地 样品编号 结构
类型拉曼光谱
峰强度比
I502/I465(%)拉曼光谱
峰面积比
A502/A465(%)红外光谱
强度比
I1095/I1157内蒙古
阿拉善盟
地区ALS-1 纤维状 15.29 15.87 1.746 ALS-2 纤维状 17.27 18.54 1.789 ALS-CH-1 纤维状 16.25 16.47 1.749 ALS-MNS-1 纤维状 19.54 19.63 1.874 ALS-MNS-2 纤维状 14.68 15.24 1.772 ALS-MNS-3 纤维状 16.00 16.13 1.743 ALS-BLMN-1 纤维状 16.54 17.21 1.759 ALS-BLMN-2 纤维状 20.37 22.00 1.724 ALS-KD1-1 纤维状 16.19 16.35 1.837 ALS-KD1-2 粒状 15.30 15.94 1.783 ALS-MNT-1 粒状 12.75 12.50 1.714 ALS-MNT-2 粒状 0 0 1.508 安徽霍山 HSY-60 粒状 2.92 - 1.722 TB1235 粒状 0 0 1.628 广西贺州 HZ-C-030 粒状 2.54 - 1.512 市场购买
(产地不详)AGATE-20 纤维状 16.30 17.62 1.793 AGATE-21 纤维状 17.13 17.93 1.830 AGAM10018 纤维状 4.62 - 1.602 AGAM10019 纤维状 0 0 1.605 注:I502/I465和A502/A465的数据均为每件样品20个测试点的平均
值。个别样品由于502 cm-1特征峰过小,无法准确计算出峰面
积,在表中以“-”表示。将表 1中可进行峰形拟合的样品的I502/I465和A502/A465进行对比可知,两种计算方法得出的结果均相对偏差在3%以内,并且其变化趋势基本相同。因此,考虑到研究样品中的斜硅石含量较低,对斜硅石的502 cm-1特征峰较小的部分样品进行峰形拟合时存在一定困难且误差较大,本文将以拉曼光谱的502 cm-1/465 cm-1峰强度比I502/I465来表示斜硅石含量相对大小的变化。
2.1.2 红外漫反射光谱分析
斜硅石在石英质玉石中的相对含量变化同样可对红外漫反射光谱产生影响。Hardgrove(2013)[12]对含有不同比例斜硅石的石英质玉石样品进行红外漫反射光谱分析,发现1095 cm-1与1157 cm-1两处谱线强度比(记作I1095/I1157)会随着斜硅石含量的改变而发生明显的变化。本文对19件研究样品进行红外漫反射光谱测试并计算其红外漫反射光谱强度比值 I1095/I1157(表 1),再将样品测试数据投点绘制成“红外漫反射光谱 I1095/I1157与拉曼光谱I502/I465散点分布图”(图 1)。由图 1可知,当拉曼光谱中的502 cm-1与465 cm-1峰强度比I502/I465>12%时,红外漫反射光谱中的I1095/I1157比值与其呈较明显的正相关关系;而当502 cm-1与465 cm-1两峰的强度比I502/I465<5%时,两者的相关性较弱。
2.1.3 X射线衍射分析
斜硅石的主要特征d值为4.43、4.35、3.11、2.88[7]。根据布拉格公式λ=2dsinθ,上述各d值对应的2θ分别为19.92°、20.27°、28.72°、30.97°。
根据拉曼光谱和红外漫反射光谱的测试结果,选取7件斜硅石相对含量不同的典型样品切除围岩并研磨成74 μm以下的粉末,进行X射线粉晶衍射测试(图 2)。由图 2可知,样品中斜硅石的衍射峰强度普遍较弱,说明样品中斜硅石的含量普遍较低。一些研究[9, 12]通过对比两种计算结果发现,拉曼光谱测试所获得的斜硅石含量更接近于真实值,并认为这种差异可能是因为X射线衍射对于纳米尺度的斜硅石无法产生连续的布拉格散射,而拉曼光谱的微小光斑对纳米尺度的斜硅石的短程有序结构反应灵敏所致。由于样品的X射线衍射分析获得的斜硅石的衍射峰普遍较弱,因此本文主要采用拉曼光谱的计算结果来表征样品中斜硅石相对含量的变化。
![]() 图 2 不同斜硅石含量样品的X射线粉晶衍射图谱:(a)斜硅石衍射峰未出现或不明显; (b)斜硅石衍射峰较为明显Q—α-石英衍射峰; M—斜硅石衍射峰。Figure 2. XRD patterns of typical samples with different moganite contents: (a) XRD patterns with less obvious or without diffraction peaks of moganite; (b) XRD patterns with relatively obvious diffraction peaks of moganite
图 2 不同斜硅石含量样品的X射线粉晶衍射图谱:(a)斜硅石衍射峰未出现或不明显; (b)斜硅石衍射峰较为明显Q—α-石英衍射峰; M—斜硅石衍射峰。Figure 2. XRD patterns of typical samples with different moganite contents: (a) XRD patterns with less obvious or without diffraction peaks of moganite; (b) XRD patterns with relatively obvious diffraction peaks of moganite2.2 斜硅石相对含量与石英质玉石结晶度的关系
X射线衍射技术同样可以用来测量矿物的结晶度。结晶度可以描述为结晶的完全程度或完整程度,其中结晶的完全程度是指物质中非晶体与晶体的相对比例,而结晶的完整程度是指物质中内部质点排列的规则程度以及结构缺陷的多少[24]。本次研究样品的X射线粉晶衍射图谱中无非晶体散射峰存在,所以本文所指的结晶度主要是石英质玉石结晶完整程度的体现。已有研究[25, 26]表明,X射线粉晶衍射图谱中67°~69°范围内的五指衍射峰可用于计算石英质玉石的结晶度指数(CI),计算公式为:CI=10·F·a/b。式中的a、b值可由位于67.74°附近、对应d(2132)=0.13844 nm衍射峰测量得出(图 3);F为比例因子,不同衍射仪的F值一般不同,需要用标准样品进行标定(一般为纯净无杂质的人工水晶,其CI值为10)。本文所选用的标准样品是由一个无色透明、内部基本无任何包裹体的水晶研磨而成的粉末。根据上述公式计算,得出本次结晶度计算公式中比例因子F=1.179。7件典型样品的结晶度指数见图 4中各点的纵坐标。
结合拉曼光谱和X射线粉晶衍射的计算结果,分别对7件X射线衍射测试样品的502 cm-1/465 cm-1谱峰强度比I502/I465和石英质玉石结晶度指数进行投点,绘制了“I502/I465-石英结晶度指数”散点分布图(图 4)。结果显示,无论是粒状结构还是纤维状结构的石英质玉石,其中斜硅石的相对含量与样品结晶度的关系有着共同的规律,即502 cm-1/465 cm-1峰强度比值(I502/I465)越大,斜硅石的相对含量越高,样品的结晶度越低。通过Excel软件对数据进行线性回归分析,石英质玉石的结晶度指数(Y)与502 cm-1/465 cm-1峰强度比值(X)之间的变化规律大致符合负相关关系:Y=-0.36X+6.93,相关系数r=-0.94。
2.3 斜硅石相对含量对石英质玉石结晶度影响的理论解释
石英质玉石中斜硅石的相对含量与石英质玉石结晶度之间的负相关关系,可以从晶体结构的角度进行解释。斜硅石的晶体结构可以看作是由左形α-石英与右形α-石英的(1011) 面晶格面网平行斜硅石晶体(101) 面交替堆叠而成,而自然界中无双晶存在的石英大多全部是由左形α-石英或右形α-石英组成。由此推测,尺度大多为纳米级的斜硅石微晶在α-石英中的出现可以视作一种结构缺陷,在一定程度上破坏了α-石英中质点规则排列所形成的长程有序结构,使以α-石英为主要成分的石英质玉石的结晶完整程度降低,从而使体现这种完整程度的结晶度指数下降。因此,图 4中能够表征斜硅石相对含量的I502/I465比值越高,其石英质玉石的结晶度指数越低。
石英质玉石结晶度指数的变化也可以通过X射线衍射图谱直观地表现出来。由于斜硅石相对含量的不同,石英质玉石中α-石英晶体结构的畸变程度也有所差异,这种结构畸变将导致在X射线衍射过程中,本应产生的衍射转化为不同程度的弥散散射。因此,图 3中斜硅石相对含量较高的样品,其衍射峰一般窄且尖锐(如样品TB1235),反之则宽且弥散(如样品ALS-MNS-1)。
不同石英质玉石中斜硅石相对含量的差异可能与其形成环境密切相关。目前,关于α-石英中斜硅石的形成仍存在较多争议,但多数认为斜硅石是在α-石英结晶的过程中形成的,而α-石英既可从流体中直接结晶,也可通过“opal-A→opal-CT/opal-C→隐晶质石英”这一序列相变形成[2, 5, 20, 27, 28]。在α-石英结晶过程中,受温度、介质的pH值、SiO2含量、杂质的种类和含量的变化等多种因素影响[1, 16, 19, 20],α-石英的结晶过程可能会发生改变,左形α-石英与右形α-石英沿(1011) 面方向的晶格面网沿一定方向交替堆叠排列,从而促成了斜硅石的结晶和生长。
3. 结论
在利用无损测试方法分析石英质玉石中斜硅石的相对含量方面,现有研究大多依靠拉曼光谱和红外光谱,而对石英结晶度的定量分析则主要借助X射线衍射技术。本文利用拉曼光谱、红外光谱和X射线衍射这三种宝玉石检测领域较为常用的测试方法,对我国4个代表性产地产出的不同颜色、不同结构类型的石英质玉石中斜硅石的相对含量差异进行了探讨,并在对比分析斜硅石相对含量与石英质玉石结晶度的过程中发现了两者之间的负相关关系,认为拉曼光谱中能够描述斜硅石相对含量变化的502 cm-1/465 cm-1两峰强度比值(X)与石英质玉石的结晶度指数(Y)的负相关关系大致符合线性方程Y=-0.36X+6.93(r=-0.94)。这一结论建立了斜硅石相对含量与石英质玉石结晶度两个不同研究方向的关联性,为判断石英质玉石的结晶程度提供了一种无损的检测方法,也为定性评价石英质玉石的品级、探讨不同产地石英质玉石的形成环境提供了新的借鉴依据。
值得指出的是,由于研究样品数量有限,还需更多的石英质玉石样品的测试数据,以期完善斜硅石的相对含量与石英质玉石结晶度之间的定量关系。
-
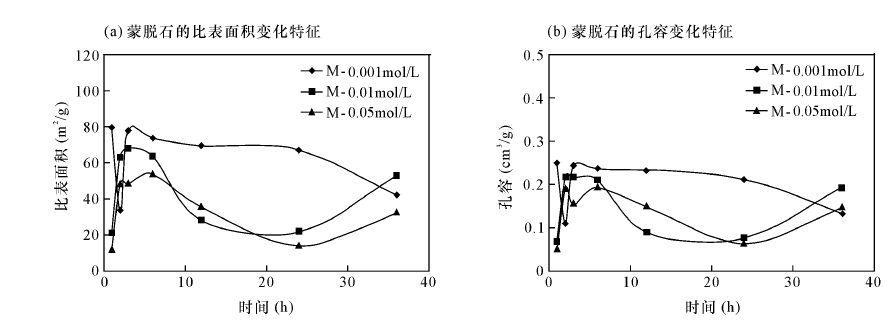
表 1 比表面积分析数据
Table 1 Analysis data of specific surface area
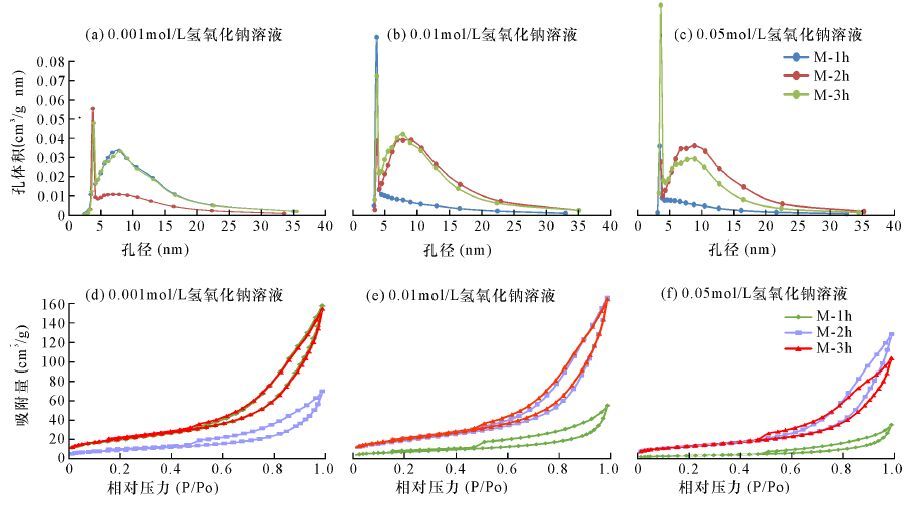
蒙脱石样品 编号 氢氧化钠 溶液浓度 (mol/L) 反应时间 (h) 孔容 (mL/g) 蒙脱石比表面积(m2/g) 各孔径段比表面积(m2/g) 各孔径段比表面积所占比例(%) >50 nm (大孔) 2~50 nm (中孔) <2 nm (微孔) >50 nm (大孔) 2~50 nm (中孔) <2 nm (微孔) M1-1 0.001 1 0.25 78.50 3.06 73.89 1.54 3.9 94.12 1.96 M1-2 0.001 2 0.11 33.01 1.75 29.75 1.51 5.3 90.12 4.58 M1-3 0.001 3 0.24 77.80 3.1 72.65 2.04 3.98 93.38 2.64 M1-4 0.001 6 0.24 73.76 3.13 68.86 1.76 4.24 93.36 2.4 M1-5 0.001 12 0.23 69.52 3.09 64.83 1.6 4.44 93.25 2.31 M1-6 0.001 24 0.21 67.05 2.58 63.35 1.11 3.85 94.48 1.67 M1-7 0.001 36 0.13 42.34 2.04 38.74 1.56 4.82 91.5 3.68 M2-1 0.01 1 0.07 21.72 1.54 18.65 1.53 7.09 85.87 7.04 M2-2 0.01 2 0.22 63.42 2.9 59.42 1.11 4.57 93.69 1.74 M2-3 0.01 3 0.22 67.95 2.76 63.3 1.9 4.06 93.16 2.88 M2-4 0.01 6 0.21 63.51 2.66 59.78 1.07 4.2 94.13 1.67 M2-5 0.01 12 0.09 28.00 1.49 25.39 1.12 5.32 90.68 4 M2-6 0.01 24 0.08 21.66 1.43 19.68 0.55 6.6 90.86 2.54 M2-7 0.01 36 0.19 52.30 2.87 47.46 1.98 5.49 90.75 3.78 M3-1 0.05 1 0.05 12.11 1.16 10.89 0.06 9.58 89.92 0.5 M3-2 0.05 2 0.19 48.69 2.61 46.08 0 5.36 94.64 0 M3-3 0.05 3 0.16 48.28 2.09 44.58 1.6 4.33 92.33 3.31 M3-4 0.05 6 0.19 53.72 2.38 51.34 0 4.43 95.57 0 M3-5 0.05 12 0.15 35.51 2.27 33.24 0 6.39 93.61 0 M3-6 0.05 24 0.06 13.96 1.48 12.48 0 10.6 89.4 0 M3-7 0.05 36 0.15 32.08 2.58 29.5 0 8.04 91.96 0 -
邱国清.储层敏感性机理及防治对策研究[J].中外能源,2014,19(6):47-50. http://www.cnki.com.cn/Article/CJFDTOTAL-SYZW201406012.htm Qiu G Q.Mechanism and Countermeasures for Prevention of Reservoir Sensitivity[J].Sino-Global Energy,2014,19(6):47-50. http://www.cnki.com.cn/Article/CJFDTOTAL-SYZW201406012.htm
李颖莉,蔡进功.泥质烃源岩中蒙脱石伊利石化对页岩气赋存的影响[J].石油实验地质,2014,36(3):352-358. http://www.cnki.com.cn/Article/CJFDTOTAL-SYSD201403015.htm Li Y L,Cai J G.Effect of Smectite Illitization on Shale Gas Occurrence in Argillaceous Source Rocks[J].Petroleum Geology and Experiment,2014,36(3):352-358. http://www.cnki.com.cn/Article/CJFDTOTAL-SYSD201403015.htm
俞杨烽,康毅力,游利军,等.碱液侵蚀:一种泥页岩井壁失稳新机理[J].石油学报,2013,34(5):983-988. http://www.cnki.com.cn/Article/CJFDTOTAL-SYXB201305023.htm Yu Y F,Kang Y L,You L J,et al.Alkali Corrosion:A New Mechanism of Shale Borehole Instability[J].Acta Petrolei Sinica,2013,34(5):983-988. http://www.cnki.com.cn/Article/CJFDTOTAL-SYXB201305023.htm
朱云,曹维政,鲁安怀,等.储层中蒙脱石碱溶相变缩膨实验研究[J].矿物学报,2011,31(1):88-94. http://www.cnki.com.cn/Article/CJFDTOTAL-KWXB201101012.htm Zhu Y,Cao W Z,Lu A H,et al.An Experimental Study on Phase Transformation of Montmorillonite in Reservoirs by Using Alkaline Treatment[J].Acta Mineralogica Sinica,2011,31(1):88-94. http://www.cnki.com.cn/Article/CJFDTOTAL-KWXB201101012.htm
陈宝,张会新,陈萍.高碱溶液对高庙子膨润土侵蚀作用的研究[J].岩土工程学报,2013,35(1):181-186. http://www.cnki.com.cn/Article/CJFDTOTAL-YTGC201301022.htm Chen B,Zhang H X,Chen P.Erosion Effect of Hyper-alkaline Solution on Gaomiaozi Bentonite[J].Chinese Journal of Geotechnical Engineering,2013,35(1):181-186. http://www.cnki.com.cn/Article/CJFDTOTAL-YTGC201301022.htm
何代平,林培滋,丁云杰,等.ASP驱油过程中蒙脱石的碱溶规律研究[J].石油与天然气化工,2001,30(3):135-137. http://www.cnki.com.cn/Article/CJFDTOTAL-STQG200103010.htm He D P,Lin P Z,Ding Y J,et al.Research on the Interactions of Montmorillonite with Alkaline Solution[J].Chemical Engineering of Oil and Gas,2001,30(3):135-137. http://www.cnki.com.cn/Article/CJFDTOTAL-STQG200103010.htm
熊生春,刘卫东,巩永刚,等.蒙脱石与碱性驱替剂反应室内实验研究[J].辽宁工程技术大学学报(自然科学版),2009,28(1):73-75. http://www.cnki.com.cn/Article/CJFDTOTAL-FXKY2009S1024.htm Xiong S C,Liu W D,Gong Y G,et al.Study on Reaction between Montmorillonite and Alkaline Flooding Agent[J].Journal of Liaoning Technical University (Natural Science Edition),2009,28(1):73-75. http://www.cnki.com.cn/Article/CJFDTOTAL-FXKY2009S1024.htm
林培滋,曾理,何代平,等.碱与岩石矿物组分(蒙脱石、石英)的相互作用及动力学研究[J].石油与天然气化工,2002,31(3):144-145. http://www.cnki.com.cn/Article/CJFDTOTAL-STQG200203012.htm Lin P Z,Zeng L,He D P,et al.Reaction and Its Kinetics of Montmorillonite,Quarts with Alkaline Solution[J].Chemical Engineering of Oil and Gas,2002,31(3):144-145. http://www.cnki.com.cn/Article/CJFDTOTAL-STQG200203012.htm
祖凯,郭建春.蒙脱石与土酸、多氢酸反应对比实验研究[J].油田化学,2012,29(1):83-85. http://www.cnki.com.cn/Article/CJFDTOTAL-YJHX201201019.htm Zu K,Guo J C.Comparative Experimental Study on Reaction of Montmorillonite with Mud Acid and Multi-Hydrogen Acid[J].Oilfield Chemistry,2012,29(1):83-85. http://www.cnki.com.cn/Article/CJFDTOTAL-YJHX201201019.htm
时佃海.王庄油田强水敏性稠油油藏热采开发研究[J].地层学杂志,2007,31(2):605-624. http://www.cnki.com.cn/Article/CJFDTOTAL-DCXZ2007S2027.htm Shi D H.Research on Thermal Recovery of Strong Water Sensitive Heavy Oil Reservoir in the Wangzhuang Oil Field[J].Journal of Stratigraphy,2007,31(2):605-624. http://www.cnki.com.cn/Article/CJFDTOTAL-DCXZ2007S2027.htm
郭欣欣,刘立,曲希玉,等.碱性地层水对火山碎屑岩改造作用的实验研究[J].石油实验地质,2013,35(3):314-319. http://www.cnki.com.cn/Article/CJFDTOTAL-SYSD201303017.htm Guo X X,Liu L,Qu X Y,et al.Experimental Study on Reformation of Volcanic Clastic Rocks by Alkaline Formation Water[J].Petroleum Geology and Experiment,2013,35(3):314-319. http://www.cnki.com.cn/Article/CJFDTOTAL-SYSD201303017.htm
赵思远,廖昕,巫锡勇,等.酸性水对黑色页岩及其风化产物溶蚀效应数值解析[J].西南大学学报(自然科学版),2013,35(12):123-130. http://www.cnki.com.cn/Article/CJFDTOTAL-XNND201312022.htm Zhao S Y,Liao X,Wu X Y,et al.Numerical Analysis of Precipitation-Dissolution of Black Shale in Acid Water and Its Weathering Products[J].Journal of Southwest University (Natural Science Edition),2013,35(12):123-130. http://www.cnki.com.cn/Article/CJFDTOTAL-XNND201312022.htm
王广华,赵静,张凤君,等.砂岩储层中CO2-地层水-岩石的相互作用[J].中南大学学报(自然科学版),2013,44(3):1167-1173. http://www.cnki.com.cn/Article/CJFDTOTAL-ZNGD201303047.htm Wang G H,Zhao J,Zhang F J,et al.Interactions of CO2-Brine-Rock in Sandstone Reservoir[J].Journal of Central South University(Science and Technology),2013,44(3):1167-1173. http://www.cnki.com.cn/Article/CJFDTOTAL-ZNGD201303047.htm
余永宁,毛卫民编著.材料的结构[M].北京:冶金工业出版社,2001:15-30. Yu Y N,Mao W M.The Structure of the Material[M].Beijing:Metallurgical Industry Press,2001:15-30.
李冰,周剑雄,詹秀春.无机多元素现代仪器分析技术[J].地质学报,2011,85(11):1878-1916. http://www.cnki.com.cn/Article/CJFDTOTAL-DZXE201111009.htm Li B,Zhou J X,Zhan X C.Modern Instrumental Analysis of Inorganic Multi-elements[J].Acta Geologica Sinica,2011,85(11):1878-1916. http://www.cnki.com.cn/Article/CJFDTOTAL-DZXE201111009.htm
曲希玉,刘立,马瑞,等.CO2流体对岩屑长石砂岩改造作用的实验[J].吉林大学学报(地球科学版),2008,38(6):959-964. http://www.cnki.com.cn/Article/CJFDTOTAL-CCDZ200806008.htm Qu X Y,Li L,Ma R,et al.Experiment on Debris-Arkosic Sandstone Reformation by CO2 Fluid[J].Journal of Jilin University (Earth Science Edition),2008,38(6):959-964. http://www.cnki.com.cn/Article/CJFDTOTAL-CCDZ200806008.htm
刘国恒,高潇玉,陈常超.陆相页岩非晶态二氧化硅定量分析新方法探索[J].高校地质学报,2015,21(3):471-477. http://www.cnki.com.cn/Article/CJFDTOTAL-GXDX201503011.htm Liu G H,Gao X Y,Chen C C.Testing a New Method for Quantitative Analysis of Amorphous SiO2 in Terrestrial Shale[J].Geological Journal of China Universities,2015,21(3):471-477. http://www.cnki.com.cn/Article/CJFDTOTAL-GXDX201503011.htm
木士春.矿物储氢研究[J].大地构造与成矿学,2005,29(1):122-130. http://www.cnki.com.cn/Article/CJFDTOTAL-DGYK20050100F.htm Mu S C.Hydrogen Storage of Minerals[J].Geotectonica et Metallogenia,2005,29(1):122-130. http://www.cnki.com.cn/Article/CJFDTOTAL-DGYK20050100F.htm
朱晓军,蔡进功.泥质烃源岩的比表面与有机质关系研究进展及意义[J].石油与天然气地质,2012,33(3):375-384. http://www.cnki.com.cn/Article/CJFDTOTAL-SYYT201203008.htm Zhu X J,Cai J G.Progress and Significance of Research on Relation between Specific Surface Area and Organic Matter in Argillaceous Source Rocks[J].Oil and Gas Geology,2012,33(3):375-384. http://www.cnki.com.cn/Article/CJFDTOTAL-SYYT201203008.htm
钟山,孙世群,陈天虎,等.盐酸酸溶对蒙脱石结构的影响[J].硅酸盐学报,2006,34(9):1162-1166. http://www.cnki.com.cn/Article/CJFDTOTAL-GXYB200609029.htm Zhong S,Sun S Q,Chen T H,et al.Influence of Hydrochloric Acid-dissolution on the Structure of Montmorillonite[J].Journal of the Chinese Ceramic Society,2006,34(9):1162-1166. http://www.cnki.com.cn/Article/CJFDTOTAL-GXYB200609029.htm
路长春,陆现彩,刘显东,等.基于探针气体吸附等温线的矿物岩石表征技术Ⅳ:比表面积的测定和应用[J].矿物岩石地球化学通报,2008,27(1):28-34. http://www.cnki.com.cn/Article/CJFDTOTAL-KYDH200801004.htm Lu C C,Lu X C,Liu X D,et al.The Technique of Surface Characteristics of Mineral Material Based on Probe Gas Adsorption Isotherm Ⅳ:Measurement and Application of Specific Surface Area[J].Bulletin of Mineralogy,Pegrology and Geochemistry,2008,27(1):28-34. http://www.cnki.com.cn/Article/CJFDTOTAL-KYDH200801004.htm
刘培生.多孔材料比表面积和孔隙形貌的测定方法[J].稀有金属材料与工程,2006,35(2):25-29. http://www.cnki.com.cn/Article/CJFDTOTAL-COSE2006S2007.htm Liu P S.Determining Methods of Specific Surface Area and Pore Morphology of Porous Materials[J].Rare Metal Materials and Engineering,2006,35(2):25-29. http://www.cnki.com.cn/Article/CJFDTOTAL-COSE2006S2007.htm
张志,杜杰,朱宏志.低温氮气吸附法研究海绵钯比表面积和孔径分布[J].稀有金属,2011,35(3):411-416. http://www.cnki.com.cn/Article/CJFDTOTAL-ZXJS201103019.htm Zhang Z,Du J,Zhu H Z.Surface Area and Pore Distribution of Sponge Palladium by Low Temperature Nitrogen Adsorption Method[J].Chinese Journal of Rare Metals,2011,35(3):411-416. http://www.cnki.com.cn/Article/CJFDTOTAL-ZXJS201103019.htm
魏祥峰,刘若冰,张廷山,等.页岩气储层微观孔隙结构特征及发育控制因素——以川南-黔北XX地区龙马溪组为例[J].天然气地球科学,2013,24(5):1048-1059. http://www.cnki.com.cn/Article/CJFDTOTAL-TDKX201305025.htm Wei X F,Liu R B,Zhang T S,et al.Micro-pores Structure Characteristics and Development Control Factors of Shale Gas Reservoir:A Case of Longmaxi Formation in XX Area of Southern Sichuan and Northern Guizhou[J].Natural Gas Geoscience,2013,24(5):1048-1059. http://www.cnki.com.cn/Article/CJFDTOTAL-TDKX201305025.htm
杨绪充.试论济阳坳陷的地温场[J].华东石油学院学报(自然科学版),1985(1):14-26. http://www.cnki.com.cn/Article/CJFDTOTAL-SYDX198501001.htm Yang X C.Notes of Geothermal Field in Jiyang Depression[J].Journal of East China Petroleum Institute,1985(1):14-26. http://www.cnki.com.cn/Article/CJFDTOTAL-SYDX198501001.htm
付艳.东营凹陷盐22块砂砾岩储层预测研究[D].北京:中国石油大学,2010:6-8. http://cdmd.cnki.com.cn/Article/CDMD-10425-2010280845.htm Fu Y.Glutenite Reservoir Prediction Research in Yan22 Block of Dongying Depression[D].Beijing:China University of Petroleum,2010:6-8. http://cdmd.cnki.com.cn/Article/CDMD-10425-2010280845.htm
严继民,张培元编著.吸附与凝聚[M].北京:科学出版社,1979:85-101. Yan J M,Zhang P Y.Adsorption and Condensation[M].Beijing:Science Press,1979:85-101.
陈金妹,张健.ASAP2020比表面积及孔隙分析仪的应用[J].分析仪器,2009(3):61-64. http://www.cnki.com.cn/Article/CJFDTOTAL-FXYQ200903017.htm Chen J M,Zhang J.Application of ASAP2020 Specific Surface Area and Porosity Analyzer[J].Analytical Instrumentation,2009(3):61-64. http://www.cnki.com.cn/Article/CJFDTOTAL-FXYQ200903017.htm
Rouquerol J,Avnir D,Fairbridge C W,et al.Recommen-dations for the Characterization of Porous Solids[J].International Union of Pure and Applied Chemistry,1994,66(8):1739-1758. http://cn.bing.com/academic/profile?id=2047404361&encoded=0&v=paper_preview&mkt=zh-cn
曾维特,张金川,丁文龙,等.延长组页岩储层纳米级孔隙特征及影响因素——以鄂尔多斯盆地柳坪171井为例[J].煤炭学报,2014,39(6):1118-1126. http://www.cnki.com.cn/Article/CJFDTOTAL-MTXB201406021.htm Zeng W T,Zhang J C,Ding W L,et al.Characteristics and Influence Factors of Nanopores in Yanchang Shale Reservoir:A Case Study of Liuping-171 Well in Erdos Basin[J].Journal of Coal Science & Engineering,2014,39(6):1118-1126. http://www.cnki.com.cn/Article/CJFDTOTAL-MTXB201406021.htm
Deneele D,Cuisinier O,Hallaire V,et al.Micostructural Evolution and Physic-Chemical Behavior of Compacted Clayey Soil Submitted to an Alkaline Plume[J].Journal of Rock Mechanics and Geotechnical Engineering,2010,2(2):169-177. doi: 10.3724/SP.J.1235.2010.00169
陈宝,张会新,陈萍.碱溶液对GMZ膨润土膨胀性和渗透性的影响[J].中南大学学报(自然科学版),2013,44(2):737-742. http://www.cnki.com.cn/Article/CJFDTOTAL-ZNGD201302045.htm Chen B,Zhang H X,Chen P.Performance Alteration of Compacted GMZ Bentonite Submitted to Hyper-Alkaline Solution[J].Journal of Central South University (Science and Technology),2013,44(2):737-742. http://www.cnki.com.cn/Article/CJFDTOTAL-ZNGD201302045.htm
王茂桢,柳少波,任拥军,等.页岩气储层黏土矿物孔隙特征及其甲烷吸附作用[J].地质论评,2015,61(1):207-216. http://www.cnki.com.cn/Article/CJFDTOTAL-DZLP201501024.htm Wang M Z,Liu S B,Ren Y J,et al.Pore Characteristics and Methane Adsorption of Clay Minerals in Shale Gas Reservoir[J].Geological Review,2015,61(1):207-216. http://www.cnki.com.cn/Article/CJFDTOTAL-DZLP201501024.htm
于炳松.页岩气储层的特殊性及其评价思路和内容[J].地学前缘,2012,19(3):252-258. http://www.cnki.com.cn/Article/CJFDTOTAL-DXQY201203027.htm Yu B S.Articularity of Shale Gas Reservoir and Its Evaluation[J].Geoscience Frontiers,2012,19(3):252-258. http://www.cnki.com.cn/Article/CJFDTOTAL-DXQY201203027.htm
曹涛涛,宋之光,王思波,等.不同页岩及干酪根比表面积和孔隙结构的比较研究[J].中国科学(地球科学),2015,45(2):139-151. doi: 10.1360/zd-2015-45-2-139 Cao T T,Song Z G,Wang S B,et al.Comparative Study on Specific Surface Area and Pore Structure of Different Shale and Kerogen[J].Scientia Sinica Terrae,2015,45(2):139-151. doi: 10.1360/zd-2015-45-2-139
陈宝,张会新,陈萍.高碱性溶液对高庙子膨润土溶蚀作用的研究[J].岩石力学与工程学报,2012,31(7):1478-1483. http://www.cnki.com.cn/Article/CJFDTOTAL-YSLX201207022.htm Chen B,Zhang H X,Chen P.Geochemical Interactions between Compacted Gaomiaozi Bentonite and Hyper-alkanline Solution[J].Chinese Journal of Rock Mechanics and Engineering,2012,31(7):1478-1483. http://www.cnki.com.cn/Article/CJFDTOTAL-YSLX201207022.htm
陈萍,唐修义.低温氮吸附法与煤中微孔隙特征的研究[J].煤炭学报,2001,26(5):552-556. http://www.cnki.com.cn/Article/CJFDTOTAL-MTXB200105023.htm Chen P,Tang X Y.The Research on the Adsorption of Nitrogen in Low Temperature and Micro-pore Properties in Coal[J].Journal of Coal Science & Engineering,2001,26(5):552-556. http://www.cnki.com.cn/Article/CJFDTOTAL-MTXB200105023.htm
韩双彪,张金川,杨超,等.渝东南下寒武页岩纳米级孔隙特征及其储气性能[J].煤炭学报,2013,38(6):1038-1043. http://www.cnki.com.cn/Article/CJFDTOTAL-MTXB201306026.htm Han S B,Zhang J C,Yang C,et al.The Characteristics of Nanoscale Pore and Its Gas Storage Capability in the Lower Cambrian Shale of Southeast Chongqing[J].International Journal of Coal Science and Technology,2013,38(6):1038-1043. http://www.cnki.com.cn/Article/CJFDTOTAL-MTXB201306026.htm
蔺亚兵,贾雪梅,马东民.基于液氮吸附法对煤的孔隙特征研究与应用[J].煤炭科学技术,2016,44(3):135-140. http://www.cnki.com.cn/Article/CJFDTOTAL-MTKJ201603026.htm Lin Y B,Jia X M,Ma D M.Study and Application of Coal Pore Features Based on Liquid Nitrogen Adsorption Method[J]. Coal Science and Technology,2016,44(3):135-140. http://www.cnki.com.cn/Article/CJFDTOTAL-MTKJ201603026.htm
薛华庆,王红岩,刘洪林,等.页岩吸附性能及孔隙结构特征——以四川盆地龙马溪组页岩为例[J].石油学报,2013,34(5):826-832. http://www.cnki.com.cn/Article/CJFDTOTAL-SYXB201305003.htm Xue H Q,Wang H Y,Liu H L,et al.Adsorption Capability and Aperture Distribution Characteristics of Shales:Taking the Longmaxi Formation Shale of Sichuan Basin as an Example[J].Acta Petrolei Sinica,2013,34(5):826-832. http://www.cnki.com.cn/Article/CJFDTOTAL-SYXB201305003.htm
胡淑琼,卢祥国,苏延昌,等.碱、表面活性剂和聚合物对储层溶蚀作用及其机理研究[J].油田化学,2013,30(3):425-429. http://www.cnki.com.cn/Article/CJFDTOTAL-YJHX201303025.htm Hu S Q,Lu X G,Su Y C,et al.Dissolution Effect of Alkali,Surfactant and Polymer on Reservoir Rock[J].Oilfield Chemistry,2013,30(3):425-429. http://www.cnki.com.cn/Article/CJFDTOTAL-YJHX201303025.htm
-
期刊类型引用(6)
1. 毛永伟,孙铭霏,李钊,代淑娟,郭小飞,赵通林. 干法超细研磨中黑滑石的机械力化学效应. 金属矿山. 2023(02): 107-113 .  百度学术
百度学术
2. 苏超,蔡锦鹏,于星才,郑其方,申培伦,刘殿文. 基于工艺矿物学的硫精矿强化固液分离研究. 有色金属工程. 2023(07): 81-87 .  百度学术
百度学术
3. 蔡来星,杨田,田景春,易娟子,任启强. 致密砂岩储层中黏土矿物发育特征及其生长机理研究进展. 沉积学报. 2023(06): 1859-1889 .  百度学术
百度学术
4. 王程,冯锴,于佳乐,王李鹏. 内蒙古油砂固体颗粒的矿物组成和亲疏水性特征. 岩矿测试. 2022(02): 332-340 .  本站查看
本站查看
5. 李浩楠,任向文,宋召军,李怀明,王文宇. 多金属结核比表面积分析测试条件研究. 岩矿测试. 2021(03): 435-443 .  本站查看
本站查看
6. 徐颖,邓利蓉,芦玉峰,左联,杜广报. 热处理对柯尔碱膨润土微观结构和物化性能的影响. 岩矿测试. 2019(03): 280-287 .  本站查看
本站查看
其他类型引用(7)



 下载:
下载:




 京公网安备 11010202008159号
京公网安备 11010202008159号